Biopolymer Degradation Standards Demystified: A Scientific Guide to ASTM, ISO, and EN Protocols for Biomedical Research
This article provides a comprehensive, technical overview of international biodegradability and compostability standards (ASTM, ISO, EN) for biomedical researchers and drug development professionals.
Biopolymer Degradation Standards Demystified: A Scientific Guide to ASTM, ISO, and EN Protocols for Biomedical Research
Abstract
This article provides a comprehensive, technical overview of international biodegradability and compostability standards (ASTM, ISO, EN) for biomedical researchers and drug development professionals. It explores the foundational science behind polymer degradation, details standardized testing methodologies for in-vitro and in-vivo applications, addresses common challenges in experimental design and data interpretation, and offers a comparative analysis of validation frameworks. The guide aims to equip scientists with the knowledge to select appropriate standards, design robust studies, and ensure regulatory compliance for novel biopolymer-based medical devices and delivery systems.
The Science of Decay: Defining Biodegradability, Compostability, and Key Standards for Biomedical Polymers
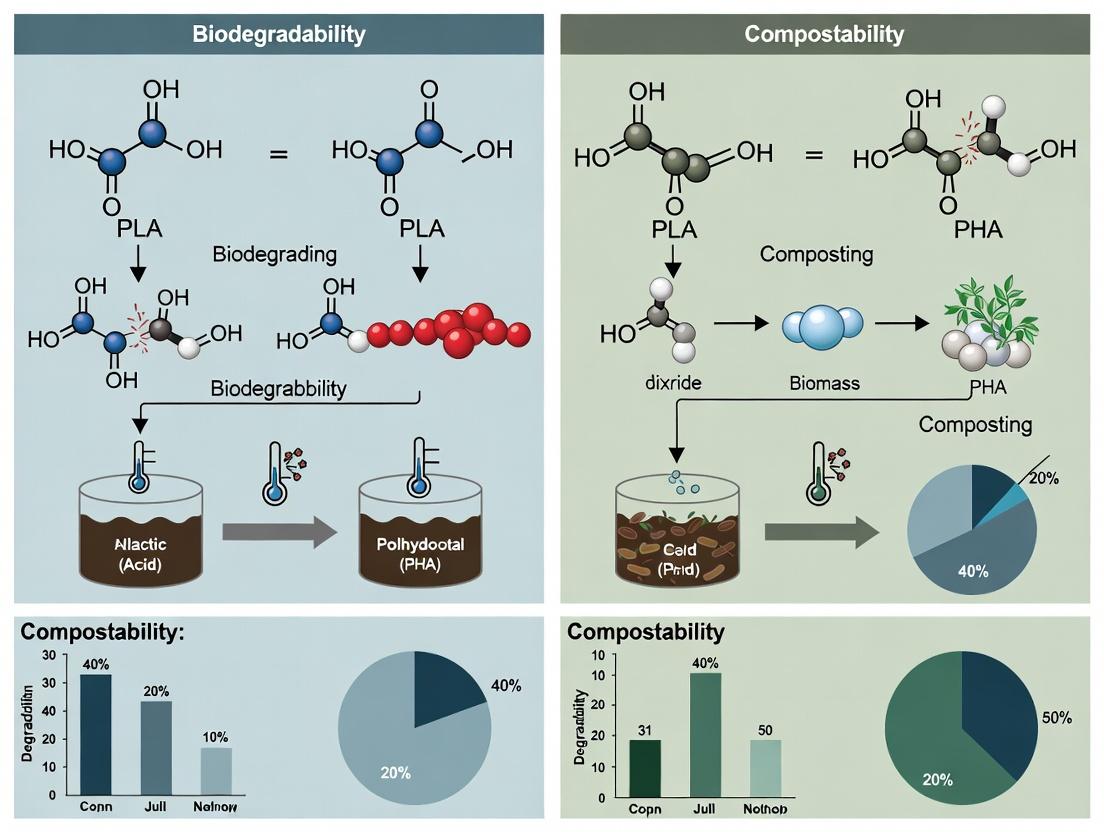
Within the context of biopolymer biodegradability and compostability standards research, precise terminology is paramount for material design, regulatory compliance, and clinical translation. In medical applications, the environmental fate of polymeric materials is a critical design parameter, yet terms like biodegradation, compostability, and bioresorption are often conflated. This technical guide delineates these core definitions, grounding them in mechanistic pathways, standardized testing protocols, and their specific implications for implantable devices, drug delivery systems, and tissue engineering scaffolds.
Core Definitions & Mechanisms
Biodegradation is the general process by which organic substances are broken down by living organisms, primarily microbial enzymes (e.g., hydrolases, oxidoreductases), into smaller molecules (oligomers, monomers), ultimately yielding carbon dioxide, methane, water, and biomass. In a medical context, this process can be mediated by both host enzymes (e.g., lysosomal hydrolases, inflammatory cell-derived reactive oxygen species) and the local microbiome. The rate and extent are highly dependent on the polymer's chemical structure, morphology, and the implantation site.
Compostability is a subset of biodegradation defined by stringent, standardized conditions. A material is compostable if it undergoes complete biological degradation in an industrial composting environment within a specified timeframe, leaving no toxic residues and integrating into the final compost. Key parameters include temperature (typically 58±2°C), relative humidity, pH, and a specific microbial ecosystem. This term is rarely applicable to in vivo medical contexts but is crucial for the end-of-life management of single-use medical products and packaging.
Bioresorption (or bioresorbability) refers to the in vivo process by which a material degrades and its degradation products are subsequently metabolized or cleared from the implantation site via natural pathways (e.g., the Krebs cycle, renal excretion). The critical distinction from general biodegradation is the focus on the complete elimination of the material from the body, with the degradation kinetics ideally matched to the healing or therapeutic timeframe (e.g., bone regeneration).
Quantitative Comparison of Standards & Metrics
The following table summarizes key quantitative parameters from leading international standards governing these processes, highlighting their distinct requirements.
Table 1: Quantitative Requirements from Key Standards
| Standard | Scope / Definition | Test Environment | Timeframe | Minimum Mineralization (CO₂ evolution) | Toxicity / Residue Requirement | Relevant Medical Context |
|---|---|---|---|---|---|---|
| ISO 14855-1 | Aerobic biodegradation under controlled composting | Industrial compost (58°C) | ≤ 6 months | 90% of positive control (e.g., cellulose) | N/A (companion tests) | Disposable items, packaging |
| ASTM D6400 | Compostability of plastics | Industrial compost | ≤ 84 days | 90% of theoretical CO₂ | ≤10% of original dry weight; compost quality tests | Sutures, single-use device components |
| ISO 10993-13 | Degradation of polymeric medical devices | In vitro simulated physiological solutions (pH 7.4, 37°C) | Device-specific | Mass loss, molecular weight change | Identification/quantification of leachables | All implantable polymers |
| ISO 13781 | Bioresorbability of polymers & implants (in vivo) | In vivo implantation (rat, rabbit, etc.) | Healing period (e.g., 12-24 months) | Not measured; focus on mass loss & tissue response | Histopathology: no chronic inflammation, normal healing | Orthopedic fixation, cardiac scaffolds |
Experimental Protocols for Assessment
Protocol A: In Vitro Enzymatic Degradation (Simulating Biodegradation)
- Objective: To assess polymer susceptibility to hydrolytic and enzymatic cleavage under simulated physiological conditions.
- Materials: Polymer specimens (e.g., PLLA, PGA films), phosphate-buffered saline (PBS, pH 7.4), enzyme solution (e.g., Proteinase K for polyesters, lysozyme for polyurethanes), incubator/shaker at 37°C.
- Methodology:
- Weigh and measure initial dimensions of sterile specimens (M₀).
- Immerse specimens in PBS (control) and PBS with specific enzyme concentration (e.g., 1 mg/mL Proteinase K).
- Incubate at 37°C with constant agitation (e.g., 60 rpm).
- At predetermined timepoints (e.g., 1, 4, 12 weeks), remove specimens (n=3-5 per group), rinse, dry to constant weight, and reweigh (Mₜ).
- Analyze mass loss (% = [(M₀ - Mₜ)/M₀] x 100), molecular weight change (via GPC), and surface morphology (via SEM).
Protocol B: In Vivo Bioresorption Assessment
- Objective: To evaluate the rate of degradation, tissue response, and ultimate clearance of an implant.
- Materials: Sterile polymer implant (e.g., porous scaffold), animal model (e.g., rat subcutaneous or bone defect model), histological stains (H&E, Masson's Trichrome).
- Methodology:
- Surgically implant the material into the target site following IACUC-approved procedures.
- Euthanize animals at multiple timepoints (e.g., 2, 6, 12, 24, 52 weeks).
- Excise the implant with surrounding tissue en bloc.
- Process for histology: fix, embed, section, and stain.
- Analyze using histomorphometry: residual implant area, thickness of fibrous capsule, presence/type of inflammatory cells (neutrophils, macrophages, giant cells), evidence of new tissue formation (bone, collagen).
Visualizing Pathways & Workflows
Title: Pathways Leading to Implant Bioresorption
Title: Bioresorption Assessment Experimental Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Degradation & Bioresorption Studies
| Reagent / Material | Supplier Examples | Function in Research |
|---|---|---|
| Poly(L-lactide) (PLLA) Standards | Corbion, Evonik, Sigma-Aldrich | Gold-standard bioresorbable control material with well-characterized degradation profile for in vitro and in vivo benchmarking. |
| Proteinase K (from Tritirachium album) | Thermo Fisher, Roche, Qiagen | Broad-spectrum serine protease used in in vitro assays to simulate aggressive enzymatic degradation of polyesters (e.g., PLA, PGA). |
| Phosphate Buffered Saline (PBS), pH 7.4 | Gibco, Sigma-Aldrich | Standard physiological medium for hydrolytic degradation studies, maintaining iso-osmotic conditions. |
| Simulated Body Fluid (SBF) | BioXtra, ChemCruz | Ion concentration solution resembling human blood plasma, used to assess bioactivity and degradation in biomimetic conditions. |
| Histology Kits (H&E, TRAP, Masson's) | Abcam, Sigma-Aldrich, Vector Labs | For staining tissue sections post-explantation to evaluate inflammatory response, cell infiltration, and tissue remodeling around degrading implants. |
| Gel Permeation Chromatography (GPC) Kits | Agilent, Waters, Tosoh Bioscience | For precise measurement of changes in polymer molecular weight and distribution over time, a critical marker of chain scission during degradation. |
| Reactive Oxygen Species (ROS) Assay Kits | Abcam, Cayman Chemical, Cell Signaling | To quantify oxidative stress (e.g., H₂O₂, O₂⁻) at the implant-tissue interface, a key driver of oxidative biodegradation pathways. |
This whitepaper provides a technical analysis of the primary degradation pathways for prominent synthetic and natural biopolymers, framed within ongoing research into biodegradability and compostability standards. Understanding the interplay between hydrolytic and enzymatic mechanisms is critical for material design in biomedical, packaging, and pharmaceutical applications.
Degradation Pathways: Core Mechanisms
Biopolymer breakdown occurs via two principal routes: abiotic hydrolysis and enzymatic depolymerization. The dominant pathway depends on polymer chemistry, morphology, and environmental conditions.
- Hydrolytic Degradation: An abiotic, water-driven scission of ester, amide, or other hydrolyzable bonds in the polymer backbone. The process is chemically defined, influenced by pH, temperature, and crystallinity. It is the primary pathway for synthetic polyesters like PLA and PGA in sterile environments.
- Enzymatic Degradation: A biologically catalyzed process where specific enzymes (e.g., proteases, lipases, depolymerases) bind to the polymer surface, facilitating cleavage. This pathway is dominant for natural polymers like PHA and is highly sensitive to the biological milieu (e.g., microbial community, enzyme kinetics).
Comparative Analysis of Biopolymer Degradation
The following table summarizes key characteristics and quantitative degradation data for common biopolymers under standardized conditions.
Table 1: Degradation Pathways & Kinetics of Common Biopolymers
| Biopolymer | Full Name | Primary Degradation Pathway (Initial) | Key Enzyme(s) (if applicable) | Typical Degradation Timeframe* | Critical Factors Influencing Rate |
|---|---|---|---|---|---|
| PLA | Poly(lactic acid) | Bulk Hydrolysis (ester bonds) | Proteinase K (in vitro) | 6-24 months (in vivo) | Molecular weight, D/L ratio, crystallinity (>50% slows rate) |
| PGA | Poly(glycolic acid) | Bulk Hydrolysis (ester bonds) | N/A (highly hydrophilic) | 3-6 months (in vivo) | High crystallinity, but high hydrophilicity accelerates hydrolysis |
| PHA (e.g., PHB) | Polyhydroxyalkanoates | Surface Enzymatic | PHA depolymerases | 3-12 months (compost) | Monomer composition (C4 vs C5), crystallinity, depolymerase specificity |
| PCL | Poly(ε-caprolactone) | Bulk/Surface Hydrolysis | Lipases (e.g., from Candida antarctica) | 24-48 months | Low Tg, semi-crystalline; enzymatic rate >> abiotic hydrolysis |
| Chitosan | Chitosan | Enzymatic | Lysozyme, chitosanases | Variable (days-weeks) | Degree of deacetylation (DDA), molecular weight, pH |
*Timeframe is highly environment-dependent (e.g., industrial compost, marine soil, physiological conditions).
Experimental Protocols for Degradation Studies
Protocol 1: In Vitro Hydrolytic Degradation (ASTM F1635)
- Objective: To measure abiotic hydrolysis under simulated physiological conditions.
- Method: Pre-weighed polymer films/cylinders (n=5) are immersed in phosphate-buffered saline (PBS, 0.1M, pH 7.4) or buffers at varying pH. Containers are placed in a shaking incubator at 37°C ± 1°C.
- Sampling: At predetermined intervals, samples are removed, rinsed with deionized water, and vacuum-dried to constant weight.
- Analysis: Mass loss (%) is calculated. Gel Permeation Chromatography (GPC) determines molecular weight decline, and Scanning Electron Microscopy (SEM) examines surface morphology.
Protocol 2: Enzymatic Degradation Assay
- Objective: To quantify enzymatic surface erosion.
- Method: Polymer films are immersed in a Tris-HCl buffer (e.g., 0.1M, pH 7.4) containing a purified enzyme (e.g., Proteinase K for PLA, PHA depolymerase for PHB). Enzyme concentration is standardized (e.g., 1 mg/mL). Controls use heat-inactivated enzyme.
- Incubation: Samples are incubated with gentle agitation at the enzyme's optimal temperature (typically 37°C).
- Analysis: Samples are processed as in Protocol 1. The rate of erosion is calculated from mass loss over time. Michaelis-Menten kinetics can be applied to determine Vmax and Km for the enzyme-polymer system.
Diagram: Decision Logic for Dominant Degradation Pathway
The Scientist's Toolkit: Key Research Reagents & Materials
Table 2: Essential Reagents for Biopolymer Degradation Studies
| Reagent/Material | Function/Application | Key Consideration |
|---|---|---|
| Phosphate Buffered Saline (PBS), pH 7.4 | Standard medium for simulating physiological hydrolytic degradation. | Use antimicrobial agents (e.g., NaN3) for abiotic-only studies. |
| Proteinase K (from Tritirachium album) | Serine protease for in vitro enzymatic degradation studies of PLA. | Activity is calcium-dependent; verify buffer compatibility. |
| PHA Depolymerase (purified) | Enzyme for specific degradation of polyhydroxyalkanoate films. | Specificity varies (e.g., PHB vs. PHBV); source from relevant microbes. |
| Lipase (e.g., from Candida antarctica) | Catalyst for enzymatic degradation of PCL and other aliphatic polyesters. | Immobilized form (CAL-B) often used for controlled reactions. |
| Lysozyme | Glycoside hydrolase for studying chitosan degradation. | Activity is pH and ionic strength dependent; mimics physiological activity. |
| Size-Exclusion/GPC Columns | For monitoring molecular weight distribution changes during degradation. | Use appropriate solvent (e.g., HFIP for PLA, Chloroform for PHA). |
| Simulated Compost/Soil Media | For mesophilic/thermophilic degradation studies per ASTM D5338. | Requires controlled moisture (58%) and temperature (58°C for thermophilic). |
The validation of biopolymer biodegradability and compostability is critically dependent on standardized testing protocols. Research in this domain requires a precise understanding of the organizations that develop these standards—primarily ASTM International, the International Organization for Standardization (ISO), and the European Committee for Standardization (CEN). Their governing principles of consensus, transparency, and global relevance directly shape the experimental frameworks used by scientists to assess material performance, ensure reproducibility, and support regulatory submissions.
ASTM International
- Governing Principles: Operates on a voluntary consensus process. Standards are developed by technical committees comprising producers, users, consumers, and general interest parties (e.g., academics, government). Decisions require a ballot process, ensuring no single interest group dominates.
- Scope & Relevance: Globally recognized, with a strong historical presence in materials science (e.g., plastics). Its standards are often adopted or harmonized with other regional systems.
- Key Biopolymer Standards: ASTM D6400 (Specification for Compostable Plastics), ASTM D6868 (Labeling of End Items), ASTM D5338 (Aerobic Biodegradation Under Controlled Composting Conditions).
International Organization for Standardization (ISO)
- Governing Principles: A network of national standards bodies (one per member country). Standards are developed through technical committees via a multi-stage process (Proposal, Preparatory, Committee, Enquiry, Approval, Publication) emphasizing global consensus and country-level ratification.
- Scope & Relevance: Provides internationally harmonized standards, crucial for global trade and research collaboration. ISO standards are often adopted by CEN through the Vienna Agreement.
- Key Biopolymer Standards: ISO 17088 (Specifications for compostable plastics), ISO 14855 (Determination of ultimate aerobic biodegradation under controlled composting conditions), ISO 20200 (Simulation of disintegration under laboratory-scale composting conditions).
European Committee for Standardization (CEN)
- Governing Principles: Comprises national standardization bodies of European Union and EFTA countries. Operates under a formal mandate system from the European Commission. Standards (EN standards) are automatically adopted as national standards by all member bodies, who must withdraw any conflicting national standards.
- Scope & Relevance: Regional but highly influential due to EU regulatory frameworks (e.g., Packaging and Packaging Waste Directive). Key for market access in Europe.
- Key Biopolymer Standards: EN 13432 (Requirements for packaging recoverable through composting and biodegradation – the foundational EU standard), EN 14995 (Plastics – Evaluation of compostability).
Comparative Analysis of Key Governing Principles and Operations
| Feature | ASTM International | ISO | CEN |
|---|---|---|---|
| Primary Governance Principle | Voluntary Consensus | International Consensus | Formal EU/EFTA Mandate & National Adoption |
| Membership | Individual members (organizations, persons) | National standards bodies (e.g., ANSI, BSI) | National standards bodies of European countries |
| Development Process | Technical Committee Balloting | Multi-stage (ISO/DIS, FDIS) | CEN Enquiry & Formal Vote (Weighted) |
| Geographic Focus | Global, strong US base | Global | Regional (European) |
| Key Output for Biopolymers | ASTM D6400, D5338 | ISO 17088, ISO 14855 | EN 13432, EN 14995 |
| Regulatory Linkage | Referenced in policies, often informational | Internationally harmonized | Directly linked to EU legislation and directives |
| Harmonization Status | Often forms basis for ISO/CEN work | Many standards parallel or derived from ASTM | Many EN standards are identical to ISO (e.g., EN ISO 14855) |
Experimental Protocols for Key Cited Standards
Protocol 1: Determination of Ultimate Aerobic Biodegradation (ASTM D5338 / ISO 14855)
- Objective: Measure the percentage of carbon in test material converted to CO₂ under controlled composting conditions.
- Methodology:
- Test Material Preparation: Material is ground to <250 µm particle size. A known mass, providing 100-400 mg of organic carbon, is mixed with mature, stable inoculum derived from compost.
- Reactor Setup: Mixture is placed in biometer flasks or respirometers maintained at 58°C ± 2°C (thermophilic conditions). Positive control (cellulose) and negative control (blank inoculum) are run concurrently.
- Measurement: Evolved CO₂ is trapped in an alkali solution (e.g., NaOH) and quantified by titration, or measured continuously via gas analysis. Duration is until a plateau in CO₂ evolution is reached, typically a maximum of 6 months.
- Calculation: Biodegradation % = [(Total CO₂ from test material – Total CO₂ from blank) / (Theoretical CO₂ production of test material)] x 100. Cellulose must demonstrate >70% biodegradation for test validity.
Protocol 2: Disintegration Test (EN 13432 / ISO 20200)
- Objective: Assess the physical fragmentation of material during composting, with no visible remnants.
- Methodology:
- Test Compost Preparation: A synthetic solid waste mixture is prepared according to a defined recipe (e.g., sawdust, rabbit food, starch, sugar, oil, urea).
- Sample Introduction: Test material is placed in a carrier bag (e.g., PE net) and mixed into the compost in a laboratory-scale reactor.
- Composting Cycle: Reactors are maintained at 58°C for 45 days, with periodic aeration and moisture adjustment. The compost is then matured at 20-25°C for an additional 15-30 days.
- Screening & Assessment: After the total cycle, contents are sieved over a 2.0 mm sieve. The mass of test material residues >2.0 mm is determined. Disintegration is considered satisfactory if less than 10% of the original dry mass remains as oversize fragments.
Visualizing the Standards Development and Experimental Pathways
Diagram 1: Standards Development & Testing Pathway
Diagram 2: Aerobic Biodegradation Test Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
| Reagent/Material | Function in Protocol | Key Specification/Standard |
|---|---|---|
| Mature Compost Inoculum | Provides the active microbial consortium for biodegradation. Must be stable, with low endogenous respiration. | Sourced from organic waste; pH 7-9; volatile solids content >30%. |
| Microcrystalline Cellulose | Positive control reference material. Validates the activity of the microbial inoculum. | Particle size <20 µm; Biodegradation must reach >70% for test validity. |
| Sodium Hydroxide (NaOH) Solution | Absorbs evolved CO₂ in respirometric methods for subsequent quantification. | Typically 0.05-1.0 N, prepared with CO₂-free water. |
| Barium Chloride (BaCl₂) | Used in titration method to precipitate carbonate from NaOH trap, allowing back-titration. | Analytical grade, 0.5-1.0 M solution. |
| Synthetic Compost Mix (ISO 20200) | Defined medium for disintegration testing. Ensures reproducibility of compost matrix. | Composition: 40% sawdust, 30% rabbit food, 10% starch, etc. |
| Carrier Bag (PE Net) | Holds test material during disintegration test, allowing microbial access while enabling recovery for sieving. | Mesh size ~1-2 mm, inert material (e.g., polyethylene). |
Within the rigorous framework of biopolymer biodegradability and compostability standards research, the precise quantification of material fate is paramount. This technical guide details the critical parameters of degradation, mineralization, and disintegration, which serve as the definitive metrics for assessing a material's environmental compatibility. For researchers and drug development professionals, particularly those engaged in designing biodegradable medical devices or excipients, these standardized measurements provide the empirical foundation for claims of compostability and environmental safety. Accurate determination under standardized conditions ensures data comparability, regulatory compliance, and scientific validity.
Core Parameters and Definitions
Degradation: An irreversible process leading to a significant change in the chemical structure of a material, typically resulting in chain scission and loss of properties. Measured via molecular weight reduction or mass loss.
Mineralization: The ultimate stage of biodegradation where the organic carbon in the material is completely converted into inorganic products (CO₂, CH₄, H₂O, and mineral salts) under the action of microorganisms. It is the key indicator of complete assimilation into natural cycles.
Disintegration: The physical breakdown of a material into tiny fragments, visually disappearing from the compost. It is a prerequisite for efficient biodegradation but does not guarantee complete mineralization.
Standardized Test Conditions & Quantitative Benchmarks
The following table summarizes core international standards and their quantitative pass/fail criteria for compostability.
Table 1: Key International Standards and Pass Criteria for Compostability
| Standard | Degradation/Mineralization Requirement | Disintegration Requirement | Ecotoxicity Requirement |
|---|---|---|---|
| ISO 17088 (ASTM D6400) | ≥90% absolute or ≥90% of reference material's mineralization (CO₂ evolution) within 180 days. | ≤10% residue >2mm after 84 days in pilot-scale test. | No adverse effects on compost quality; regulated heavy metals below limits. |
| EN 13432 | ≥90% absolute or ≥90% of reference material's mineralization (CO₂ evolution) within 6 months. | ≤10% residue >2mm after 12 weeks (pilot-scale). | Pass germination and plant growth tests; regulated elements below limits. |
| ISO 14855-1 (Controlled Composting) | ≥90% of reference material's mineralization (CO₂ evolution) within 180 days. | Not directly assessed in this respirometric test. | Not part of this specific method. |
Table 2: Typical Degradation Data for Common Biopolymers Under Controlled Composting (ISO 14855)
| Biopolymer | Time to 90% Mineralization (days) | Final Mineralization at 180 days (%) | Notes |
|---|---|---|---|
| Microcrystalline Cellulose (Reference) | ~45 | >95 | Readily degradable positive control. |
| Poly(lactic acid) - PLA | 90-120 | ~90-95 | Requires specific compost conditions (thermophilic). |
| Poly(hydroxyalkanoates) - PHA | 80-100 | ~95 | Highly compostable. |
| Starch-Polyester Blends | 70-110 | 85-98 | Rate depends on starch content and polyester type. |
| Poly(butylene adipate-co-terephthalate) - PBAT | 100-150 | ~90 | Degrades well in compost. |
Detailed Experimental Protocols
Protocol for Aerobic Biodegradation (Mineralization) - ISO 14855-1
Objective: To determine the ultimate aerobic biodegradability of plastic materials under controlled composting conditions by measuring evolved carbon dioxide.
Materials:
- Reactor vessels (2-5 L) with air supply and CO₂-trapping columns.
- Mature, stable inoculum derived from compost.
- Test material and reference material (cellulose), ground to <250 µm.
- Solid compost substrate (e.g., synthetic food waste, sawdust).
- CO₂ trapping solution (e.g., 0.5-1.0 N NaOH or Ba(OH)₂).
- Titration setup (for NaOH method) or conductivity meter.
Methodology:
- Preparation: Mix the test material (typically 100-200g total organic carbon) thoroughly with the compost inoculum and substrate in the reactor. Moisture content is adjusted to 50-55%. A blank (only inoculum and substrate) and a reference (with cellulose) are prepared in parallel.
- Incubation: Reactors are placed in a thermostatically controlled environment at 58°C ± 2°C. Humified air is continuously blown through the reactors.
- CO₂ Trapping & Measurement: The evolved CO₂ is carried by the effluent air into trapping columns containing the alkaline solution. The amount of CO₂ is determined at regular intervals (e.g., daily or weekly) by titrating the excess base with HCl or by measuring conductivity change.
- Calculation: The cumulative amount of CO₂ produced from the test material is calculated by subtracting the CO₂ from the blank. The percentage biodegradation (mineralization) is calculated as: (CO₂ from test - CO₂ from blank) / (Theoretical CO₂ from test material) * 100.
- Duration: The test typically runs for a maximum of 6 months, or until a plateau is reached.
Protocol for Disintegration - ISO 20200
Objective: To evaluate the disintegration of plastic materials under simulated composting conditions.
Materials:
- Reactor containers (e.g., 2-5 L).
- Solid synthetic compost matrix (based on ISO 20200: sawdust, rabbit food, compost, corn starch, sugar, oil, urea).
- Mature compost inoculum.
- Test material in the form of film or pieces (typically 1x1 cm to 2x2 cm).
- Sieves with 2.0 mm and 0.7 mm mesh.
Methodology:
- Preparation: The test material pieces are carefully weighed and placed into labeled synthetic compost matrix bags. These bags are mixed into the main compost matrix in the reactor. Moisture is maintained at ~55%.
- Composting: Reactors are kept in a controlled environment with periodic temperature cycling (e.g., 35-58°C) to mimic thermophilic phases. The compost is manually turned and re-moistened at regular intervals (e.g., weekly).
- Sampling & Retrieval: At predetermined time points (e.g., 4, 8, 12 weeks), the entire contents of a reactor are emptied. The compost is carefully sieved through a 2.0 mm sieve.
- Analysis: The material retained on the 2.0 mm sieve is collected, cleaned, dried, and weighed. The degree of disintegration is calculated as: (Initial dry mass - Recovered dry mass) / (Initial dry mass) * 100.
- Criteria: To pass, ≥90% of the test material must disintegrate (i.e., fragment to pieces <2mm) within the test period.
Visualization of Pathways and Workflows
Title: Biopolymer Composting Degradation Pathway
Title: ISO 14855 Mineralization Test Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials and Reagents for Biodegradation Testing
| Item / Reagent | Function / Purpose | Key Consideration |
|---|---|---|
| Mature Compost Inoculum | Source of microbial consortium for biodegradation. Must be validated for activity. | Critical for reproducibility. Often defined by standard (e.g., pH, C/N ratio, respiration activity). |
| Microcrystalline Cellulose (Avicel PH-105) | Positive reference material for mineralization tests. | Serves as the benchmark (100% biodegradable) to normalize test results and validate inoculum activity. |
| Sodium Hydroxide (NaOH) 0.5-1.0N | CO₂ trapping solution in respirometric tests. | Concentration must be precise. Requires careful titration with standardized HCl. |
| Barium Hydroxide (Ba(OH)₂) | Alternative CO₂ trapping solution. Allows for conductimetric measurement. | Faster, in-line measurement possible. Forms precipitate (BaCO₃). |
| ISO 20200 Synthetic Compost Matrix | Standardized solid medium for disintegration and lab-scale composting tests. | Provides consistent nutrient and physical background, eliminating variability of natural compost. |
| 2.0 mm & 0.7 mm Test Sieves | For retrieving and grading non-disintegrated material remnants. | Mesh size defined by standards (e.g., EN 13432 uses 2.0 mm). |
| Controlled Environment Chamber | Maintains precise thermophilic temperature (58°C) for composting tests. | Temperature uniformity and stability are critical for test validity. |
| Automatic Respirometer System (e.g., OxiTop, Sapromat) | Automated measurement of O₂ consumption or CO₂ production. | Increases throughput and data point density, reducing manual labor. |
This technical guide, framed within a broader thesis on biopolymer standards research, examines the critical distinction and intersection between environmental degradation (environmental fate) and physiological breakdown (clinical fate) of biomaterials. For researchers and drug development professionals, understanding how standardized in-vitro tests predict complex in-vivo behavior is paramount for designing safe, effective, and sustainable medical products.
Biopolymers used in medical applications (e.g., drug-eluting implants, tissue scaffolds) must satisfy two distinct fate requirements:
- Environmental Fate: The material's degradation profile under conditions such as industrial composting (e.g., ASTM D6400, ISO 14855) or marine environments. Key metrics include disintegration, ultimate biodegradation to CO₂, water, and biomass, and ecotoxicity.
- Clinical Fate: The material's behavior in the physiological environment (hydrolysis, enzymatic cleavage, phagocytosis) and its local/systemic biological response (biocompatibility per ISO 10993).
The core challenge is that standard environmental tests are poor predictors of clinical fate, and vice-versa, necessitating a tailored battery of analyses.
Comparative Analysis of Standard Test Frameworks
The following tables summarize key quantitative parameters and endpoints from major standard testing regimes.
Table 1: Core Standards for Environmental vs. Clinical Fate Assessment
| Aspect | Environmental Fate (e.g., Compostability) | Clinical Fate (Biocompatibility) |
|---|---|---|
| Governing Standards | ASTM D6400, ISO 14855, EN 13432 | ISO 10993 series, USP <151> |
| Primary Degradation Driver | Microbial enzymatic activity | Hydrolysis, enzymatic (e.g., lysozyme) activity, cellular phagocytosis |
| Key Quantitative Endpoints | - Disintegration: >90% pass 2mm sieve- Biodegradation: >90% conversion to CO₂- Ecotoxicity: Germination/Growth >90% of control | - Cytotoxicity: Cell viability >70% (ISO 10993-5)- Hemolysis: <5% (ISO 10993-4)- Pyrogenicity: Pass LAL or MAT test |
| Timeframe | 180 days (typical for compost) | Days to years (depends on application) |
| Final Environment | Compost, soil, marine | Extracellular fluid, lysosomal compartments, phagosomes |
Table 2: In-Vitro Hydrolytic Degradation: Comparing Simulated Environments
| Test Condition (Buffered Solution) | pH | Temperature | Primary Simulating | Typical Sampling Intervals | Key Analytical Endpoints |
|---|---|---|---|---|---|
| ISO 14855 (Controlled Composting) | ~7.5 (dynamic) | 58°C ± 2°C | Thermophilic compost | 10, 45, 90, 180 days | CO₂ Evolution, Molecular Weight (Mw) Loss |
| ISO 10993-13 (Degradation of Polymers) | 7.4 ± 0.2 | 37°C ± 1°C | Physiological fluid | 1, 3, 7, 28, 56+ days | Mass Loss, Mw Loss, Monomer Release (HPLC) |
| Simulated Lysosomal Fluid | 4.5 - 5.0 (with enzymes) | 37°C ± 1°C | Intracellular phagolysosome | 1, 7, 24, 72 hours | Mass Loss, Mw Loss, Osmolarity Change |
Experimental Protocols for Predictive Analysis
To bridge the gap between standard tests and in-vivo performance, the following integrated protocol is recommended.
Protocol: Tiered Hydrolytic and Enzymatic Degradation Screening
Objective: To characterize a biopolymer's degradation profile across environmentally and clinically relevant conditions in parallel.
Materials & Reagents:
- Phosphate Buffered Saline (PBS), 0.1M, pH 7.4: Simulates extracellular fluid.
- Citrate-Phosphate Buffer, 0.1M, pH 5.0: Simulates acidic inflammatory or lysosomal environments.
- Proteinase K or Lysozyme Solution: For enzymatic degradation studies (clinical fate).
- Cellulase/Lipase Cocktail: For enzymatic degradation studies (environmental fate).
- Sterile, Pyrogen-Free Containers: Essential for clinical fate simulations.
- Gel Permeation Chromatography (GPC/SEC) System: For monitoring molecular weight changes.
- UPLC/HPLC with UV/RI Detectors: For quantification of degradation products (monomers, oligomers).
Procedure:
- Sample Preparation: Precisely cut or weigh sterile polymer samples (n=5 per group). Record initial dry mass (M₀) and package for sterile transfer if required.
- Immersion: Aseptically immerse samples in the following solutions:
- Group A: PBS, pH 7.4, 37°C (Clinical: hydrolysis).
- Group B: PBS + 1 mg/mL Lysozyme, pH 7.4, 37°C (Clinical: enzymatic).
- Group C: Citrate Buffer, pH 5.0, 37°C (Clinical: acidic hydrolysis).
- Group D: Compost Simulant Buffer, pH 8.0, 58°C (Environmental: thermophilic).
- Group E: Compost Buffer + Enzyme Cocktail, 58°C (Environmental: enzymatic).
- Incubation: Place containers on an orbital shaker (50 rpm) in temperature-controlled incubators. Maintain sterility for Groups A-C.
- Sampling: At predetermined intervals (e.g., 1, 7, 28, 56 days), remove samples in triplicate.
- Analysis:
- Rinsing & Drying: Rinse samples with deionized water and dry to constant mass (Mₜ). Calculate mass loss:
(M₀ - Mₜ)/M₀ * 100%. - Molecular Weight: Dissolve a portion of the dried polymer and analyze via GPC.
- Degradant Analysis: Filter the incubation medium and analyze via UPLC for specific monomers or acids (e.g., glycolic acid, lactic acid).
- pH Monitoring: Record pH of the medium at each interval.
- Rinsing & Drying: Rinse samples with deionized water and dry to constant mass (Mₜ). Calculate mass loss:
Protocol: IntegratedIn-VitroBiocompatibility & Environmental Toxicity
Objective: To assess biological response to degradation products from both clinical and environmental perspectives.
Materials & Reagents:
- L929 Mouse Fibroblast Cell Line: Standard for cytotoxicity (ISO 10993-5).
- Lemna minor (Duckweed) or Lactuca sativa (Lettuce) Seeds: Standard for plant growth inhibition tests (environmental ecotoxicity).
- AlamarBlue or MTT Reagent: For metabolic cytotoxicity assay.
- Hemoglobin Standard & Cyanmethemoglobin Reagent: For hemolysis assay (ISO 10993-4).
- Hoagland's Growth Medium: For plant toxicity tests.
Procedure:
- Extract Preparation: Prepare extraction mediums per ISO 10993-12: a) Cell culture medium with serum (37°C for 24h), b) Deionized water (50°C for 72h) for environmental assessment.
- Cytotoxicity (Clinical Fate):
- Culture L929 cells in 96-well plates.
- Expose to serial dilutions of the clinical extract (cell culture medium).
- After 24-72h, add AlamarBlue and measure fluorescence. Calculate cell viability relative to negative control.
- Hemocompatibility (Clinical Fate):
- Dilute fresh human or animal blood with PBS.
- Incubate with polymer samples or clinical extracts.
- Centrifuge and measure hemoglobin release spectrophotometrically. Calculate % hemolysis.
- Phytotoxicity (Environmental Fate):
- Place Lemna minor fronds or Lactuca sativa seeds in multi-well plates with Hoagland's medium.
- Add the environmental extract (aqueous) at varying concentrations.
- After 7 days, measure frond count/biomass or seed germination/root length. Calculate inhibition %.
Visualizing Relationships and Workflows
Title: Dual Fate Assessment Workflow for Biopolymers
Title: Key Degradation Pathways Driving Clinical Fate
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Dual-Fate Biopolymer Research
| Item | Function | Example Supplier/Cat. No. (Illustrative) |
|---|---|---|
| Lysozyme (from chicken egg white) | Enzyme for simulating enzymatic hydrolysis of certain polyesters (e.g., PLLA) in physiological conditions. | Sigma-Aldrich, L6876 |
| Proteinase K | Broad-spectrum serine protease for aggressive enzymatic degradation studies. | Thermo Fisher Scientific, AM2546 |
| Cellulase from Trichoderma reesei | Enzyme cocktail for simulating microbial degradation of cellulose-based biopolymers. | Megazyme, E-CELTR |
| AlamarBlue Cell Viability Reagent | Fluorescent redox indicator for high-throughput cytotoxicity screening per ISO 10993-5. | Thermo Fisher Scientific, DAL1025 |
| Pyrogen-Free Water | Essential for preparing extracts for in-vivo tests and hemocompatibility to avoid confounding results. | MilliporeSigma, W1503 |
| USP Reference Standard for Lactic Acid | Quantification of degradation products from PLA polymers via HPLC/UPLC. | USP, 100700 |
| Simulated Body Fluid (SBF) | Ionic solution approximating human blood plasma for bioactivity and degradation studies. | Fisher Scientific, 50-753-6034 |
| Lemna minor Growth Kit | Standardized test organism for assessing environmental ecotoxicity of degradation products. | Carolina Biological Supply, 156470 |
| GPC/SEC Columns (e.g., PLgel) | Columns for accurate molecular weight distribution analysis of degrading polymers. | Agilent Technologies, PL1110-6300 |
| Sterile, Pyrogen-Free Vials/Containers | For all sample preparation and incubation in clinical fate studies to prevent contamination and pyrogen introduction. | Thermo Fisher Scientific, 02-912-196 |
Protocols in Practice: A Step-by-Step Guide to ASTM D6400, ISO 14855, and Related Testing Methodologies
Within the critical research on biopolymer biodegradability and compostability standards, selecting the appropriate test method is foundational. This guide provides a decision framework and technical protocols for four key methods, aiding researchers and scientists in generating valid, comparable data.
Key Criteria Comparison Table
The following table summarizes the quantitative requirements and outputs for each standard.
| Test Parameter | ASTM D5338 (Industrial Composting) | ISO 17556 / ASTM D5988 (Soil Burial) | ISO 14851/14852 (Aquatic, Aerobic) | ASTM D5511 / ISO 15985 (Anaerobic Digestion) |
|---|---|---|---|---|
| Test Environment | Controlled compost, thermophilic conditions | Natural or synthetic soil, mesophilic | Aerated aqueous medium, mesophilic | Anaerobic sludge or digestate, mesophilic/thermophilic |
| Temperature | 58 ± 2 °C | Typically 20-28 °C | 20-25 °C | 35 ± 2 °C (mesophilic) or 50 ± 2 °C (thermophilic) |
| Duration | Up to 180 days | Up to 2 years (often 6-24 months) | Typically up to 60 days | Up to 30-45 days |
| Measured Output | CO₂ evolution | CO₂ evolution (or O₂ uptake) | O₂ consumption or CO₂ evolution | Biogas (CH₄ + CO₂) volume and composition |
| Pass/Fail Criterion (for 90% biodegradation) | ≥ 90% of theoretical CO₂ within 180 days | ≥ 90% in positive control soil (time varies) | ≥ 90% of theoretical O₂/CO₂ | ≥ 70% of theoretical biogas yield (often vs. cellulose control) |
| Control Material | Microcrystalline cellulose | Cellulose powder or Whatman filter paper | Sodium benzoate or microcrystalline cellulose | Microcrystalline cellulose (50-70% biodegradation expected) |
| Inoculum Source | Mature, stabilized compost from MSW or yard waste | Active soil from field (e.g., 0-20 cm depth) | Activated sludge from WWTP (or marine source) | Digested sludge from anaerobic reactor |
Carbon Conversion & Data Validity Table
| Metric | ASTM D5338 | Soil Burial | Aquatic | Anaerobic |
|---|---|---|---|---|
| Theoretical Maximum CO₂ (mg) | Calculated from sample carbon content | Calculated from sample carbon content | Calculated from sample carbon content | N/A |
| Minimum Inoculum Activity | Must show ≥ 70% degradation of control in 45 days | Requires validation of soil microbial activity (e.g., respiration) | Endogenous respiration < 60 mg O₂/L·h | Control cellulose must show characteristic degradation curve |
| Reference Material Degradation (%) | Cellulose: 70% ± 20% in 45 days | Cellulose: >70% in 6 months (site-specific) | Sodium Benzoate: >60% in 14 days | Cellulose: >70% of theoretical biogas in 15 days |
| Toxicity Check Required? | Yes (comparison of control vs. control + test material) | Optional, but recommended | Yes (inhibition test with e.g., 3,5-DCP) | Yes (via parallel reactor with toxicant) |
Detailed Experimental Protocols
ASTM D5338: Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions
Principle: The test material is mixed with inoculum derived from matured compost and incubated under thermophilic conditions. The evolved CO₂ is trapped and quantified; biodegradation is calculated as the percentage of theoretical CO₂ yield based on the sample's carbon content.
Protocol:
- Inoculum Preparation: Sieve matured compost (particle size < 10 mm). Adjust moisture content to 50-55% and pH to 7-8.5. Pre-condition at 58°C for 3-5 days.
- Reactor Setup: Use vessels of at least 2L. Blend test material (typically 100-200g, ground to < 250 µm) with 600-800g of wet inoculum. A blank (only inoculum) and a control (inoculum + 10g cellulose) are prepared in parallel.
- Incubation: Incubate at 58 ± 2°C in the dark for up to 180 days. Maintain aerobic conditions by regularly flushing with CO₂-free air (e.g., 30-40 mL/min).
- CO₂ Trapping & Measurement: The effluent air is bubbled through a series of traps containing a 0.1-0.5N NaOH or Ba(OH)₂ solution. The trapped carbonate is quantified by titration with HCl or via TIC analysis at regular intervals (e.g., days 1, 3, 7, 14, 28, 45, 60, 90, 180).
- Calculation:
- Cumulative CO₂ (mg) from sample = (CO₂ from test vessel) - (Avg. CO₂ from blank vessels).
- Theoretical CO₂ (ThCO₂) from sample = (Sample mass (g)) × (% Carbon in sample/100) × (44/12).
- % Biodegradation = (Cumulative CO₂ from sample / ThCO₂) × 100.
Soil Burial Test (Based on ISO 17556/ASTM D5988)
Principle: Test material is buried in soil, and the evolved CO₂ is measured directly or via O₂ consumption. The test evaluates biodegradation in a natural, solid environment.
Protocol:
- Soil Characterization & Preparation: Collect soil (e.g., from top 20 cm of an agricultural site). Sieve (< 2 mm). Analyze for pH, moisture, total organic carbon, and nitrogen. Adjust moisture to 40-60% of water holding capacity. Pre-condition at test temperature for 2 weeks.
- Sample Preparation & Burial: Prepare samples (typically film or fragments, ~100 mg C per vessel). Place samples between two layers of nylon mesh for easy retrieval. Bury them at a depth of 2-5 cm in soil containers (e.g., 1-5 L jars).
- Incubation & Measurement: Incubate in the dark at a constant temperature (e.g., 25 ± 2°C). For respirometric methods, flush vessels periodically with CO₂-free air and measure evolved CO₂ via GC, IR, or titration of NaOH traps. Alternatively, use manometric O₂ consumption systems.
- Termination & Recovery: At test end, recover material remnants, clean, and dry to determine mass loss via gravimetry, which can correlate with respirometric data.
- Calculation: Similar to ASTM D5338, based on measured cumulative CO₂ or O₂ consumption versus theoretical demand.
ISO 14852: Determination of the Ultimate Aerobic Biodegradability in an Aqueous Medium
Principle: The test material is suspended in a mineral medium with a defined microbial inoculum (activated sludge). The oxygen demand is measured in a closed respirometer.
Protocol:
- Medium & Inoculum: Prepare a mineral salts medium (containing KH₂PO₄, K₂HPO₄, Na₂HPO₄, NH₄Cl, CaCl₂, MgSO₄, FeCl₃). Use activated sludge from a municipal plant, washed and aerated. Maintain a low biomass concentration (e.g., 30 mg/L suspended solids).
- Respirometer Setup: Fill vessels with medium, inoculum, and test material (providing 100-200 mg C/L). Include blank (inoculum only) and control (sodium benzoate, 100 mg C/L). Use a manometric or electrolytic respirometer.
- Incubation: Incubate at 20-25°C in the dark with continuous stirring. Monitor the pressure drop (O₂ consumption) or CO₂ evolution automatically.
- Calculation:
- Cumulative O₂ consumption (mg) for sample = (O₂ in test vessel) - (Avg. O₂ in blank).
- Theoretical Oxygen Demand (ThOD) = [16 × (2C + ½(H - Cl) + 3N - O)] / Molecular weight of compound.
- % Biodegradation = (Cumulative O₂ consumption for sample / ThOD) × 100.
ASTM D5511: Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under High-Solids Anaerobic-Digestion Conditions
Principle: The test material is mixed with a digesting solid waste inoculum in a reactor, and the volume and composition of produced biogas are measured.
Protocol:
- Inoculum & Substrate: Use actively digesting sludge from an anaerobic digester treating municipal solid waste. Total solids should be > 20%. The test material is ground (< 250 µm). A cellulose control is mandatory.
- Reactor Setup: Use serum bottles (e.g., 500 mL) with working volumes of ~50%. Add inoculum, test material (equivalent to 100-200 mg C), and distilled water to achieve a total solids content of ~30%. Flush headspace with N₂/CO₂ mix, seal with butyl rubber stoppers and aluminum caps.
- Incubation: Incubate at 35 ± 2°C (mesophilic) with gentle agitation. Monitor daily for pressure buildup initially.
- Biogas Measurement: Use a pressure transducer or glass syringe to measure gas volume. Periodically analyze gas composition (CH₄ and CO₂) via GC. Vent reactors as needed to prevent over-pressurization.
- Calculation:
- Cumulative Biogas (mL) from sample = (Biogas from test) - (Avg. biogas from blank).
- Convert biogas to mg of Carbon as CH₄+CO₂: Use ideal gas law and carbon content of CH₄ (12/16) and CO₂ (12/44).
- Theoretical Carbon in sample (mg) = Sample mass (mg) × (%C/100).
- % Biodegradation = (Cumulative Carbon in biogas from sample / Theoretical Carbon in sample) × 100.
Decision Framework & Workflow Visualization
Title: Decision Framework for Selecting a Biodegradation Test Method
The Scientist's Toolkit: Key Research Reagent Solutions
| Item/Category | Function & Rationale |
|---|---|
| Microcrystalline Cellulose (Avicel PH-105) | Positive Control Material. Provides a highly reproducible, >90% biodegradable carbon source to validate inoculum activity and system performance across all test types. |
| Activated Sludge (from Municipal WWTP) | Inoculum for Aquatic Tests. A complex, standardized microbial community representing the aerobic biodegradation potential in wastewater environments. Must be washed and pre-aerated. |
| Matured Compost (from MSW or Yard Waste) | Inoculum for ASTM D5338. Provides a diverse, thermophilic microbial consortium adapted to degrading complex organic matter under composting conditions. |
| Anaerobic Digester Sludge | Inoculum for ASTM D5511. A methanogenic microbial community essential for simulating high-solids anaerobic digestion processes. Activity is critical. |
| Sodium Hydroxide (NaOH) 0.1-0.5N Solutions | CO₂ Trapping & Titration. Used in respirometric methods to absorb evolved CO₂; subsequent titration quantifies the amount. Must be CO₂-free prepared. |
| Sodium Benzoate | Positive Control for Aquatic Tests. A readily soluble, completely biodegradable reference compound used to verify inoculum activity in aqueous systems (ISO 14851/14852). |
| Mineral Salts Media (e.g., ISO 14852配方) | Aqueous Test Medium. Provides essential nutrients (N, P, K, trace elements) to support microbial growth while preventing limitation, ensuring only carbon from the test material is limiting. |
| Butyl Rubber Stoppers & Aluminum Seals | Anaerobic Reactor Seals. Provide gas-tight seals for serum bottles used in anaerobic biodegradation tests, allowing for pressure buildup and gas sampling via syringe. |
| 3,5-Dichlorophenol (3,5-DCP) | Inhibition Control. Used in aquatic tests to confirm system sensitivity by showing suppressed respiration at a known toxic concentration, validating that positive results are genuine. |
| Nylon Mesh Bags (e.g., 50-100 µm pore size) | Sample Containment for Soil Burial. Allows soil microbes and moisture to interact with the test material while enabling retrieval of material fragments for gravimetric analysis. |
This technical guide details the laboratory-scale simulation of aerobic composting as prescribed by ISO 20200:2021, "Plastics — Determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test." Within a broader thesis on biopolymer biodegradability and compostability standards, this setup is the foundational experimental engine. It enables the generation of precise, reproducible data on disintegration rates, which is a critical first step before more complex and costly analyses of biodegradation (e.g., CO₂ evolution per ISO 14855) and ecotoxicity. Mastery of this protocol is essential for researchers and drug development professionals evaluating polymeric drug delivery systems, capsule materials, or other pharmaceutical plastics designed for environmental benignity.
Laboratory Equipment
The setup requires equipment to create and maintain the controlled composting environment. The following table summarizes the essential apparatus.
Table 1: Essential Laboratory Equipment for ISO 20200 Testing
| Equipment | Specification / Purpose | Key Function |
|---|---|---|
| Composting Reactors | 2-10 L volume, with gas-permeable lid (e.g., porous fabric) or air supply system. Material: inert (glass, stainless steel). | Contains the solid waste matrix and test material. Allows for aerobic conditions. |
| Climate Chamber or Incubator | Temperature control range: 35-70°C ± 2°C. Humidity control (to prevent desiccation) is advantageous. | Maintains the defined thermophilic and mesophilic temperature phases. |
| Analytical Balance | Capacity ≥ 200g, readability 0.01g. | Precisely weighs test materials, compost components, and retrieved samples. |
| pH Meter | With a sturdy, puncture-resistant electrode for semi-solid matrices. | Monitors compost pH, a critical indicator of microbial activity and process health. |
| Sieve Set | 2.0 mm mesh size (definitive) and optionally 1.0 mm. Frame material: non-corrosive (stainless steel). | Determines the degree of disintegration by separating residual test material from mature compost. |
| Drying Oven | Temperature range 50-105°C, forced ventilation recommended. | Dries compost samples and retrieved test material fragments to constant mass. |
| Homogenizer | Robust blender or mixer capable of handling dense, fibrous solids. | Prepares the initial compost inoculum and waste matrix to a uniform consistency. |
Preparation of Synthetic Solid Waste and Inoculum
ISO 20200 specifies a synthetic, well-defined solid waste matrix to ensure reproducibility between labs and over time.
Table 2: Composition of the Synthetic Solid Waste Matrix (Dry Basis)
| Component | Percentage by Mass | Preparation Note |
|---|---|---|
| Sawdust | 40% | Coniferous, particle size 0.5-1.0 cm. Air-dried. Primary source of carbon and bulk. |
| Compost (Inoculum) | 10% | Mature, stabilized compost from organic waste. Source of active microorganisms. |
| Rabbit Feed | 30% | Ground pelletized feed. Provides readily degradable organic nitrogen and nutrients. |
| Corn Starch | 10% | Industrial grade. Rapidly metabolizable carbon source to kick-start microbial activity. |
| Sucrose | 5% | Industrial grade white sugar. Easily accessible energy source for rapid microbial growth. |
| Corn Oil | 4% | Refined. Source of lipids and higher carbon-chain compounds. |
| Urea | 1% | Technical grade. Provides a concentrated, soluble nitrogen source to adjust C/N ratio. |
| Total Dry Mass | 100% |
Experimental Protocol: Matrix and Inoculum Preparation
- Drying: Dry all solid components (sawdust, rabbit feed) at 70°C until constant mass. Cool in a desiccator.
- Weighing: Precisely weigh each dry component according to the target total dry mass (e.g., 600g for a typical test).
- Dry Mixing: Homogeneously mix all dry components (sawdust, ground rabbit feed, corn starch, sucrose, urea) in a large container.
- Inoculum Addition: Add the mature compost (10% of dry mass) and mix thoroughly.
- Liquid Addition: Gradually add the corn oil and then distilled water while mixing vigorously. The target is a moisture content of 50-55% (w/w) of the total wet mixture.
- Maturation (Pre-conditioning): Place the complete wet mixture in a reactor with gas exchange in the climate chamber at 35°C for up to 4 days. This activates the microbial community. Monitor temperature daily; a rise of 10-15°C above ambient confirms active composting.
Experimental Setup and Test Execution
Workflow: ISO 20200 Disintegration Test Setup
Experimental Protocol: Test Execution
- Test Material Preparation: Cut or form test material to dimensions ≤25 x 25 x 5 mm. Dry to constant mass at 50°C or under reduced pressure. Weigh precisely (M₁).
- Loading Reactors: Mix the pre-conditioned matrix. For each test and control vessel, layer the matrix and evenly distribute the test material or positive control (cellulose paper) pieces within it. Use a minimum of 3 reactors per material.
- Incubation: Place reactors in the climate chamber. Follow the defined temperature profile:
- Thermophilic Phase: 58°C ± 2°C for 7 days.
- Mesophilic Phases: 50°C ± 2°C for 7 days, then 45°C ± 2°C for 7 days, then 35°C ± 2°C for the final 7 days. Total duration: 28 days.
- Moisture Maintenance: Weigh reactors weekly and replenish evaporated water with distilled water to maintain initial mass.
- Termination & Analysis: After 28 days, empty the entire contents of each reactor into a tray. Dry at 105°C to constant mass. Gently hand-sieve the entire dried contents over a 2.0 mm sieve. Carefully collect all visible test material fragments retained on the sieve. Weigh these dried residues (M₂).
- Calculation: Calculate the degree of disintegration, D, as: D (%) = [(M₁ - M₂) / M₁] × 100 where M₁ is the initial dry mass of test material and M₂ is the final dry mass of material retained >2.0 mm. Report the mean and standard deviation of replicate reactors.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials and Reagents for ISO 20200 Testing
| Item | Function/Justification |
|---|---|
| Mature Compost (Inoculum) | Non-sterile, sourced from organic municipal solid waste. Provides a diverse, active consortium of compost-derived microorganisms essential for a representative test. Must be sieved (<10 mm) and used fresh. |
| Positive Control (Cellulose Paper) | Whatman No. 1 filter paper or equivalent. A highly degradable reference material used to validate the activity of the compost ecosystem in each test run. Expected disintegration >90%. |
| Negative Control (Polyethylene Film) | A virgin polyethylene film (e.g., 50μm thick). A non-degradable reference material used to confirm the test's selectivity. Expected disintegration <5%. |
| Urea (CH₄N₂O) | Technical grade. A critical nitrogen source to adjust the Carbon-to-Nitrogen (C/N) ratio of the synthetic matrix to an optimal range (~25-30:1) for microbial growth. |
| Solid Waste Components | Sawdust, rabbit feed, corn starch, sucrose, corn oil. Precisely defined synthetic food waste analogues. Their consistent quality and proportions are the cornerstone of inter-laboratory reproducibility. |
| pH Buffer Solutions (pH 4.01, 7.00, 10.01) | For accurate calibration of the pH meter prior to measuring the compost matrix, as pH is a key process variable. |
Data Management and Thesis Integration
Table 4: Example Data Recording and Output Table
| Reactor ID | Material | Initial Dry Mass, M₁ (g) | Final Residue Mass >2.0mm, M₂ (g) | Disintegration, D (%) | Remarks (pH trend, visual notes) |
|---|---|---|---|---|---|
| R1 | Test Biopolymer A | 5.00 | 0.85 | 83.0 | pH dropped to 5.8 in week 1, recovered to 7.5. |
| R2 | Test Biopolymer A | 5.02 | 0.90 | 82.1 | -- |
| R3 | Test Biopolymer A | 4.98 | 0.81 | 83.7 | -- |
| Mean ± SD | Test Biopolymer A | -- | -- | 82.9 ± 0.8 | -- |
| C1 | Positive Control (Cellulose) | 2.50 | 0.05 | 98.0 | Full visual disintegration. |
| C2 | Positive Control (Cellulose) | 2.51 | 0.07 | 97.2 | -- |
| Mean ± SD | Positive Control | -- | -- | 97.6 ± 0.6 | -- |
| N1 | Negative Control (PE) | 5.10 | 5.05 | 1.0 | No visual change. |
| N2 | Negative Control (PE) | 5.07 | 5.02 | 1.0 | -- |
| Mean ± SD | Negative Control | -- | -- | 1.0 ± 0.0 | -- |
This data, generated under standardized conditions, feeds directly into a research thesis. It allows for:
- Comparative Analysis: Ranking different biopolymer formulations.
- Structure-Disintegration Relationships: Correlating material properties (crystallinity, chemical structure) with disintegration performance.
- Validation: Confirming that a material meets the ISO 20200 disintegration threshold (90% for thicker materials, as per related standards like ISO 17088) before proceeding to higher-tier tests (biodegradation, ecotoxicity). A robust laboratory setup as described is, therefore, indispensable for credible research in the field of biodegradable plastics.
Within the critical research domain of biopolymer biodegradability and compostability standards, robust quantitative analytical techniques are paramount. Validating claims of environmental benignity requires multi-faceted evidence of polymer breakdown. This technical guide details four core analytical pillars: CO2 evolution via respirometry, mass loss, molecular weight reduction, and visual disintegration. Together, these methods provide a comprehensive assessment of biodegradation kinetics, extent, and mechanism, essential for researchers and standards development.
Core Quantitative Techniques
CO2 Evolution (Respirometry)
Respirometry measures microbial metabolic activity by quantifying carbon dioxide produced from the mineralization of carbon substrates. It is the cornerstone of international standards (e.g., ISO 14855, ASTM D5338) for determining ultimate aerobic biodegradability.
Experimental Protocol (Gravimetric/Manual Method):
- Apparatus: Use glass bioreactors with a sample compartment and a separate alkaline trap (e.g., NaOH solution).
- Test Material: Precisely weigh test biopolymer (~100-200 mg carbon) and mix with mature compost inoculum (solid test) or mineral medium (liquid test).
- Incubation: Maintain under controlled compost-like conditions (e.g., 58°C ± 2°C, >50% humidity, aerobic).
- CO2 Trapping: At regular intervals, the evolved CO2 is absorbed by a known volume and concentration of barium hydroxide or sodium hydroxide in the trap.
- Titration: The unreacted hydroxide is titrated with standardized hydrochloric acid (HCl) using phenolphthalein or a potentiometric endpoint.
- Calculation: The amount of CO2 evolved is calculated from the difference in acid titer between blank and sample reactors. Biodegradation percentage is expressed as (CCO2(test) - CCO2(blank)) / Cinitial (polymer) × 100.
Automated Systems: Modern automated respirometers (e.g., OxiTop, Respicond) continuously measure pressure change or conductivity, providing real-time data.
Mass Loss
Direct measurement of the physical disappearance of test material, often coupled with respirometry or performed in simulated environments.
Experimental Protocol (Soil Burial or Compost Disintegration):
- Sample Preparation: Pre-weigh (Minitial) dried test films or pieces (e.g., 20mm x 20mm). Use a minimum of triplicates.
- Exposure: Bury samples in controlled compost or soil beds meeting standard criteria (e.g., 58°C, moisture ~50%). Include positive (cellulose) and negative (PE) controls.
- Retrieval: At predetermined time points, carefully retrieve samples, gently rinse with distilled water to remove adherent biomass and soil.
- Drying & Weighing: Dry samples to constant weight in a desiccator or low-temperature oven (e.g., 40°C). Weigh to determine final mass (Mfinal).
- Calculation: Mass Loss (%) = [(Minitial - Mfinal) / Minitial] × 100. Correct for mass loss from controls.
Molecular Weight Reduction
Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC) is used to monitor the cleavage of polymer chains, indicating abiotic or enzymatic depolymerization.
Experimental Protocol (GPC/SEC Analysis):
- Sample Extraction: At time points, extract residual polymer from compost/soil matrix using a suitable solvent (e.g., chloroform for polyesters). Filter to remove particulates.
- Solution Preparation: Precisely dissolve dried extract in the GPC eluent (e.g., THF for many bioplastics) at a known concentration (~2-5 mg/mL).
- Chromatography: Inject solution into GPC system equipped with refractive index (RI) and/or multi-angle light scattering (MALS) detectors. Use a column set calibrated with narrow polystyrene or polymethyl methacrylate standards relevant to the polymer.
- Data Analysis: Calculate average molecular weights (Mn - number average, Mw - weight average) and polydispersity index (PDI = Mw/Mn). Reduction in Mn is a key indicator of chain scission.
Visual Disintegration
A qualitative-to-semi-quantitative assessment of physical fragmentation, often the first visible sign of biodegradation, as per standards like ISO 20200.
Experimental Protocol:
- Setup: Place pre-weighed test pieces (≥ 1cm x 1cm) in mesh bags (e.g., nylon lattice) within compost.
- Monitoring: At regular intervals, retrieve bags and visually assess the state of the material under standardized lighting.
- Grading: Use a defined scale (e.g., 0-5: 0=no change, 5=complete disintegration). Photograph samples.
- Sieve Test: After a set period, contents are sieved (e.g., 2mm mesh). The fraction remaining on the sieve is dried and weighed to provide a semi-quantitative "disintegration degree."
Data Presentation
Table 1: Comparative Summary of Core Quantitative Techniques for Biopolymer Analysis
| Technique | Primary Metric | Key Apparatus | Standard Methods | Data Output | Information Provided |
|---|---|---|---|---|---|
| Respirometry | CO2 Evolution | Bioreactor, NaOH trap, titrator or pressure sensor | ISO 14855, ASTM D5338 | Cumulative CO2 (mg), % Mineralization | Ultimate aerobic biodegradability, kinetic profile. |
| Mass Loss | Residual Dry Mass | Analytical balance, controlled compost bed, desiccator | ISO 20200, ASTM D5988 | Mass Loss (%) | Physical disappearance, disintegration rate. |
| Molecular Weight (GPC) | Mn, Mw, PDI | GPC/SEC system, RI/MALS detectors, calibrated columns | N/A (Analytical technique) | Molecular weight distributions | Chain scission, depolymerization mechanism. |
| Visual Disintegration | Disintegration Grade | Mesh bags, sieves, imaging system | ISO 20200 | Grade (0-5), % Retained on Sieve | Macroscopic physical breakdown. |
Table 2: Typical Data from a Simulated PLA Biodegradation Study in Controlled Compost
| Time (Days) | Cumulative CO2 Evolution (% of Theoretical) | Mass Loss (%) | Number Avg. Mol. Wt. (Mn - kDa) | Visual Disintegration Grade (0-5) |
|---|---|---|---|---|
| 0 | 0 | 0 | 120.0 | 0 |
| 15 | 5.2 ± 1.1 | 2.5 ± 0.8 | 98.5 ± 5.2 | 1 (Slight surface erosion) |
| 30 | 25.8 ± 3.5 | 18.4 ± 2.1 | 45.2 ± 8.1 | 3 (Fragmentation, holes) |
| 45 | 78.5 ± 4.2 | 85.2 ± 3.7 | 8.5 ± 2.3 | 5 (Complete disintegration) |
| 90 | 92.1 ± 2.8 | 96.5 ± 1.5 | <5.0 | 5 (No recoverable film) |
Experimental Workflow and Logical Relationships
Diagram 1: Integrated workflow for biopolymer biodegradation analysis.
The Scientist's Toolkit: Key Research Reagent Solutions & Materials
Table 3: Essential Materials for Biodegradation Experiments
| Item / Reagent | Function / Purpose | Example / Specification |
|---|---|---|
| Mature Compost Inoculum | Source of standardized microbial consortium for biodegradation tests. | Sourced as per ISO 14855: >3 months matured, specific C/N ratio, no large inert particles. |
| Positive Control Material | Validates microbial activity of the inoculum. | Microcrystalline cellulose (e.g., Avicel PH-105), >70% mineralization expected in 45 days. |
| Negative Control Material | Checks for non-biological mass loss (e.g., leaching). | Polyethylene film or glass fiber. |
| Absorbent for CO2 (Solid Test) | Traps evolved CO2 for gravimetric measurement. | Soda lime (NaOH on carrier) or solid potassium hydroxide. |
| Absorbent Solution (Liquid Test) | Traps CO2 in aqueous systems for titration. | 0.05-0.1 N Barium Hydroxide (Ba(OH)2) or Sodium Hydroxide (NaOH). |
| Titrant Solution | Quantifies unreacted hydroxide in trap. | Standardized 0.05 N Hydrochloric Acid (HCl). |
| GPC/SEC Calibration Standards | Calibrates columns for accurate molecular weight determination. | Narrow dispersity polystyrene (PS) or polymethyl methacrylate (PMMA) standards. |
| GPC/SEC Eluent | Mobile phase for polymer dissolution and separation. | High-grade Tetrahydrofuran (THF, stabilized) with specified water content for reproducibility. |
| Extraction Solvents | Recovers residual polymer from compost matrix for analysis. | Chloroform (for polyesters like PLA/PBAT), Hexafluoroisopropanol (HFIP, for some rigid polymers). |
| Mesh Bags | Holds test material during disintegration tests, allowing microbial access. | Nylon or polyester mesh with defined aperture size (e.g., 1-2mm). |
Within the rigorous framework of biopolymer biodegradability and compostability standards research, the integrity of long-term studies hinges on robust data acquisition and strategic sampling schedules. Extended testing periods, which can span from several months to years for complete mineralization analysis, introduce significant challenges: environmental parameter drift, biological community succession, and sample degradation. This technical guide details best practices to maintain statistical power, ensure reproducibility, and uphold test validity throughout these prolonged timelines, crucial for regulatory submissions and scientific validation.
Foundational Principles for Long-Term Validity
Temporal Resolution vs. Resource Allocation: The core tension in extended studies lies between sampling frequency and practical constraints. High-frequency sampling increases data resolution but risks depleting sample mass, increasing labor, and potentially disturbing test systems (e.g., compost reactors). Low-frequency sampling may miss critical transition points, such as the onset of biofilm formation or the rapid degradation phase.
Defining Critical Control Points (CCPs): Borrowing from risk management frameworks, establishing CCPs—time points or system states where variability must be controlled—is essential. For biodegradation in soil or compost, CCPs include the stabilization of microbial activity post-setup, the depletion of a labile carbon fraction, and periods of abiotic stress (e.g., temperature fluctuations).
Best Practices in Sampling Schedule Design
A scientifically defensible schedule is neither purely arbitrary nor strictly regular. It must be adaptive to the process kinetics.
1. Phase-Dependent Scheduling: Sampling intensity should mirror the expected degradation kinetics.
- Lag Phase: Sparse sampling (e.g., weekly) to establish baseline and confirm activity initiation.
- Exponential Degradation Phase: Intensive sampling (e.g., daily or every other day) to capture rapid changes in key indicators.
- Plateau Phase: Reduced sampling (e.g., monthly) to confirm stabilization and final endpoints.
2. Trigger-Based Sampling: Supplementary to the fixed schedule, define actionable triggers for additional sampling. For example:
- If the CO₂ evolution rate deviates by >2 standard deviations from the model prediction.
- If the temperature within a compost vessel deviates from the set point by >5°C for more than 24 hours.
- If visual inspection indicates unexpected physical changes (e.g., fragmentation, discoloration).
3. Statistical Power Analysis for Schedule Definition: Prior to experiment initiation, use power analysis to determine the minimum number of replicates and sampling points required to detect a statistically significant effect (e.g., a 10% difference in mineralization rate) with a power of 0.8-0.9. This prevents under-sampling and ensures the study can meet its objectives.
Data Acquisition & Control Systems
Automated vs. Manual Data Acquisition: For high-frequency parameters (e.g., CO₂, O₂, temperature, pressure), automated, continuous data-logging systems are non-negotiable for maintaining validity. They remove human error and provide a complete temporal dataset for kinetic modeling. Manual sampling remains vital for destructive tests (e.g., molecular weight analysis, microbial diversity).
Environmental Parameter Stability: Long-term compostability studies (e.g., ISO 14855) require tight control. Data acquisition must continuously monitor and log:
- Temperature (with feedback control to composting temperatures, typically 58°C ± 2°C)
- Humidity of inlet air (maintained at >50% RH to prevent desiccation)
- Flow rate of purge air (critical for respirometric calculations)
- Pressure differentials (indicating filter or vessel blockages)
Key Experimental Protocols for Biodegradation Assessment
Protocol 1: Respirometric Measurement of Ultimate Biodegradability (Based on ISO 14855-1)
- Objective: Determine the ultimate aerobic biodegradability of a biopolymer under controlled composting conditions by measuring evolved carbon dioxide.
- Methodology:
- Test Material Preparation: Grind material to particles <250 µm. Precisely weigh a mass containing 1-2 g of organic carbon. Mix thoroughly with mature, sieved compost inoculum.
- Reactor Setup: Place mixture in a bioreactor (2-5 L) with a porous base for aeration. Connect to a continuous air supply (humidified, CO₂-free air) at a constant, measured flow rate (e.g., 50-100 mL/min).
- CO₂ Trapping & Measurement: The effluent gas from reactors (test, positive control [cellulose], and blank [compost only]) is bubbled through a series of traps containing an excess of 0.1-0.5M NaOH or Ba(OH)₂ solution.
- Sampling Schedule: The alkaline traps are replaced and titrated according to a phase-dependent schedule: Days 1, 2, 3, 5, 7, 10, 14, 21, 28, then weekly until Day 45, and biweekly until stabilization (often up to 6 months). Daily automated measurements via NDIR CO₂ sensors are strongly recommended.
- Calculation: Cumulative CO₂ evolution is calculated for test and control vessels. Biodegradation percentage = [(CO₂(test) – CO₂(blank)) / (Theoretical CO₂(test material))] * 100.
Protocol 2: Sampling for Molecular Weight Analysis During Degradation
- Objective: Track changes in polymer molecular weight (Mw, Mn) to understand degradation mechanism (bulk vs. surface erosion).
- Methodology:
- Destructive Sampling: At pre-defined CCPs (e.g., 0%, 25%, 50%, 75% of predicted mineralization, and endpoint), sacrificially remove replicate vessels or retrieve representative sub-samples.
- Polymer Recovery: Lyophilize sample. Extract polymer residues using a suitable solvent (e.g., chloroform for PLA, hexafluoroisopropanol for PHA) via Soxhlet extraction. Filter to remove residual compost/inoculum.
- Analysis: Analyze recovered polymer via Gel Permeation Chromatography (GPC/SEC) against appropriate polystyrene or polymer-specific standards.
- Schedule Rationale: Infrequent but strategically timed sampling prevents excessive disturbance and provides data points correlating structural change with respirometric output.
Table 1: Comparison of Standardized Test Durations & Key Sampling Parameters
| Test Standard | Typical Duration | Critical Sampling Parameter | Minimum Sampling Frequency (Recommended) | Acceptance Criterion for Validity (Positive Control) |
|---|---|---|---|---|
| ISO 14855-1 (Controlled Composting) | Up to 6 months | CO₂ Evolution | Daily (automated), Weekly (titration) | Cellulose biodegradation >70% at 45 days |
| ASTM D5338 (Compost) | 45-180 days | CO₂ Evolution | Every 2-3 days (manual), Continuous (automated) | Cellulose biodegradation >70% at 45 days |
| ISO 17556 (Soil) | Up to 2 years | O₂ Consumption/CO₂ Evolution | Weekly for first 3 months, then Monthly | Cellulose biodegradation >60% at end of test |
| OECD 301B (Ready Biodegradability) | 28 days (can extend to 60) | Dissolved Organic Carbon (DOC) | Days 0, 7, 14, 21, 28 (minimum) | Reference compound degradation >70% within 14 days |
Table 2: Impact of Sampling Frequency on Detected Degradation Kinetics (Theoretical Model Data)
| Sampling Interval | Detected Lag Phase (days) | Calculated Max Degradation Rate (mg C/day) | Error in Final Mineralization % (±) | Risk Profile |
|---|---|---|---|---|
| Daily | 5.0 | 12.5 | 0.5% | Low - High resolution |
| Weekly | 5.5 - 7.0 | 11.0 - 14.0 | 2-5% | Medium - May miss inflection points |
| Biweekly | 5.0 - 10.0 | 9.5 - 15.5 | 5-10% | High - High uncertainty in kinetics |
| Monthly | 5.0 - 28.0 | 8.0 - 17.0 | >15% | Unacceptable - Unreliable model fitting |
Visualizing Workflows and Relationships
Diagram 1: Adaptive Sampling Schedule Decision Workflow (100 chars)
Diagram 2: From Data Acquisition to Kinetic Parameters (97 chars)
The Scientist's Toolkit: Research Reagent & Material Solutions
Table 3: Essential Materials for Extended Biodegradation Studies
| Item | Function & Rationale | Key Consideration for Long-Term Validity |
|---|---|---|
| Mature, Sieved Compost Inoculum (ISO 14855) | Provides a diverse, active microbial community. Sieving (<10mm) ensures homogeneity. | Must be sourced from a stable, mature compost pile. Activity should be verified with a cellulose positive control in each batch. Pre-blending large batches minimizes inoculum variability. |
| Cellulose Microcrystalline (Positive Control) | Validates microbial activity and serves as a benchmark for test material degradation. | Use a defined, high-purity standard (e.g., Sigma Aldrich 310697). Particle size should match test material. |
| CO₂-Free, Humidified Air Supply | Provides O₂ for aerobic metabolism while preventing desiccation and baseline CO₂ drift. | Use a reliable CO₂ scrubber (e.g., soda lime) and a calibrated mass flow controller. Humidity probe with feedback loop is essential. |
| NaOH or Ba(OH)₂ Trapping Solution (0.1-0.5M) | For manual titration methods, quantitatively absorbs evolved CO₂ as carbonate. | Standardize solution frequently (daily for intensive sampling). Ensure excess capacity per trapping period to prevent saturation. |
| Non-Dispersive Infrared (NDIR) CO₂ Sensor | Enables continuous, real-time monitoring of CO₂ evolution in respirometric systems. | Requires regular calibration against zero gas and a known CO₂ standard. Sensor drift must be checked weekly. |
| Sterile Compostable Bags (for ASTM D6400) | Used in real compost field tests. Must be certified compostable to not interfere. | Store away from moisture and UV light. Include blank bags (no test material) to account for bag mineralization. |
| Polymer Recovery Solvents (e.g., CHCl₃, HFIP) | For extracting residual polymer from matrix for GPC/Molecular Weight analysis. | Use high-purity, anhydrous grades to prevent polymer degradation during extraction. Implement safe, vapor-containment protocols. |
| Automated Data Logger with Redundant Storage | Collects time-series data from all sensors (T, pH, CO₂, flow) without interruption. | Must have battery/UPS backup. Data should be written to two independent media (e.g., internal SD + networked PC) daily. |
Maintaining test validity over extended periods in biopolymer degradation research demands a shift from static, calendar-based sampling to a dynamic, phase-aware, and trigger-responsive strategy. Integrating robust power analysis during design, implementing continuous automated data acquisition for key parameters, and strategically scheduling destructive analyses at Critical Control Points form the triad of an effective long-term data integrity plan. By adhering to these best practices, researchers can generate kinetic data that withstands statistical scrutiny and meets the exacting requirements of international standards and regulatory bodies.
Within the critical research on Biopolymer biodegradability and compostability standards, translating material performance from controlled laboratory conditions to relevant biological environments is paramount. ASTM F1635, "Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants," serves as a cornerstone methodology. This case study positions the rigorous application of ASTM F1635 not as a standalone compliance exercise, but as an indispensable, predictive preclinical tool. It bridges the gap between fundamental material science and the complex in-vivo reality, informing the development of next-generation biodegradable medical devices.
Core Principles of ASTM F1635
ASTM F1635 provides a controlled, reproducible in-vitro environment to simulate the hydrolytic degradation mechanism predominant in many biopolymers (e.g., PLGA, PCL, PGA). Its primary objective is to generate quantitative data on mass loss, molecular weight changes, and mechanical property decay over time under simulated physiological conditions (pH 7.4, 37°C).
Detailed Experimental Protocol for Preclinical Research
The following protocol expands upon the standard for enhanced preclinical relevance.
A. Sample Preparation & Characterization (Time T0)
- Material: Fabricate test specimens (e.g., films, porous scaffolds, molded pins) per final device manufacturing processes.
- Sterilization: Apply the intended clinical sterilization method (e.g., ethylene oxide, gamma irradiation, e-beam) to account for its impact on initial polymer properties.
- Baseline Characterization:
- Dry Mass (W₀): Precisely weigh (≥ 3 samples).
- Molecular Weight: Determine via Gel Permeation Chromatography (GPC).
- Thermal Properties: Analyze via Differential Scanning Calorimetry (DSC) for crystallinity (ΔHf).
- Mechanical Properties: Perform tensile/compressive testing per relevant ASTM standards (e.g., D638, D695).
B. Degradation Study Setup
- Immersion Medium: Phosphate Buffered Saline (PBS) at pH 7.4 ± 0.1, with 0.02% sodium azide to inhibit microbial growth. For accelerated studies, use an alkaline (e.g., pH 10.0) or acidic (e.g., pH 2.0) medium.
- Volume: Maintain a sink condition (≥ 30 mL of medium per 1 g of polymer).
- Incubation: Agitate in a thermostatically controlled water bath or incubator at 37°C ± 1°C.
- Time Points: Predefined intervals (e.g., 1, 2, 4, 8, 12, 26, 52 weeks).
C. Post-Immersion Analysis at Each Time Point (T₁, T₂...Tₙ)
- Retrieval: Remove samples (n ≥ 3 per time point) from medium. Rinse with deionized water and dry to constant mass under vacuum.
- Mass Loss Measurement: Weigh dry sample (Wₜ). Calculate percentage mass remaining:
(Wₜ / W₀) * 100. - Molecular Weight Analysis: Perform GPC on degraded samples to track Mn and Mw reduction.
- Water Uptake/Porosity: Note sample physical changes. Calculate water uptake if relevant.
- Medium Analysis: Monitor pH change of the immersion medium. Analyze for soluble degradation products via HPLC or spectrophotometry.
- Mechanical Testing: Perform on wet or re-dried samples as appropriate to track property loss.
D. Data Correlation & Modeling: Fit degradation data (mass loss, Mn loss) to kinetic models (e.g., first-order, empirical algebraic) to predict long-term behavior.
Table 1: Degradation Profile of Common Biopolymers per ASTM F1635 Protocol (PBS, 37°C)
| Polymer | Initial Mn (kDa) | Time to 50% Mn Loss | Time to 10% Mass Loss | Notable Mechanical Loss (50% Strength) | Degradation Byproducts Monitored |
|---|---|---|---|---|---|
| PLGA 50:50 | 50-70 | 4-6 weeks | 8-12 weeks | 6-8 weeks | Lactic acid, Glycolic acid |
| PLGA 85:15 | 50-70 | 12-16 weeks | 24-30 weeks | 18-22 weeks | Lactic acid, Glycolic acid |
| PCL | 80-100 | >52 weeks | >52 weeks | >52 weeks | 6-hydroxycaproic acid |
| PGA | 90-110 | 6-8 weeks | 12-16 weeks | 4-6 weeks | Glycolic acid |
| PDLLA | 100-150 | 20-28 weeks | 40-52 weeks | 30-40 weeks | Lactic acid |
Table 2: Impact of Key Variables on Degradation Rate (Relative Change)
| Variable | Condition | Effect on Degradation Rate vs. Standard | Mechanistic Insight |
|---|---|---|---|
| pH | 10.0 (Alkaline) | Increase by ~200-300% | Base-catalyzed ester hydrolysis |
| pH | 2.0 (Acidic) | Increase by ~150-250% | Acid-catalyzed ester hydrolysis |
| Crystallinity | High (Annealed PLLA) | Decrease by ~60-80% | Slower water penetration |
| Porosity | High (>80%) | Increase by ~100-150% | Increased surface area for hydrolysis |
| Sterilization | Gamma Irradiation (25 kGy) | Increase by ~30-50% | Chain scission reduces initial Mn |
Visualizing the Workflow and Degradation Pathways
Title: ASTM F1635 Preclinical Degradation Workflow
Title: Hydrolytic Degradation & Autocatalysis Pathway
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Materials & Reagents for ASTM F1635 Studies
| Item | Function / Rationale | Critical Specification Notes |
|---|---|---|
| High-Purity PBS Buffer | Simulates physiological ionic strength and pH. | Sterile, endotoxin-free, without calcium/magnesium to avoid precipitation. |
| Sodium Azide (NaN₃) | Preservative to inhibit microbial growth in long-term studies. | Use at 0.02% w/v; handle as toxic. Alternative: 0.5% Chloroform-saturated PBS. |
| HPLC-Grade Water | For rinsing samples and preparing mobile phases. | Low conductivity (<1.0 µS/cm) to prevent interference in analytics. |
| GPC/SEC Standards (PMMA or PS) | For calibrating molecular weight distributions. | Narrow dispersity (Ð) standards matching polymer chemistry for accuracy. |
| pH Calibration Buffers (4.0, 7.0, 10.0) | Ensure accuracy of medium pH monitoring. | NIST-traceable, fresh solutions for reliable calibration. |
| Enzymes (e.g., Proteinase K, Lipase) | For studying enzyme-mediated degradation of specific polymers. | High-activity, purified formulations to introduce biological catalytic factors. |
| Simulated Body Fluid (SBF) | Alternative medium for bioresorbable ceramics or mineralization studies. | Ion concentration equal to human blood plasma. |
| Vacuum Desiccator | For drying samples to constant mass without thermal degradation. | Use with indicating desiccant (e.g., Drierite). |
Navigating Experimental Pitfalls: Common Challenges in Biodegradation Testing and Data Interpretation
Within the critical research on Biopolymer biodegradability and compostability standards, a fundamental challenge is the accurate assessment of true microbial metabolic activity. Standardized tests (e.g., ISO 14855, ASTM D5338) measure CO₂ evolution or O₂ consumption as proxies for mineralization. However, non-polymer constituents—such as plasticizers, stabilizers, residual monomers from synthesis, or the inherent toxicity of the test article itself—can inhibit the microbial consortia in compost or soil. This inhibition leads to falsely low biodegradation rates, misclassifying inherently biodegradable materials as recalcitrant. This whitepaper details the mechanisms, detection protocols, and mitigation strategies for these inhibition artifacts, ensuring data integrity in compliance testing and material development.
Mechanisms of Microbial Inhibition
Inhibition occurs when a chemical component interferes with the physiological processes of key decomposer microorganisms (bacteria, fungi). The primary pathways are:
- Membrane Disruption: Surfactants or hydrophobic monomers can integrate into and disrupt microbial cell membranes, causing leakage and cell lysis.
- Enzyme Inhibition: Additives like certain antimicrobials or heavy metal stabilizers can bind irreversibly to active sites of critical extracellular hydrolases (e.g., esterases, glycosidases).
- Uncoupling of Oxidative Phosphorylation: Phenolic compounds can dissipate the proton motive force across mitochondrial and bacterial membranes, reducing ATP yield.
- Genotoxicity: Residual monomers like acrylamide or vinyl chloride can alkylate DNA, halting cell division in key degraders.
Experimental Protocols for Detection & Quantification
Protocol 3.1: Tiered Inhibition Screening Assay
This protocol identifies inhibition and determines the non-inhibitory concentration of a test article leachate.
- Leachate Preparation: Prepare an aqueous extract of the test material (polymer + additives) per ISO 14855-2 (e.g., 100g material in 1L water, 24h agitation, 70°C). Filter (0.2µm).
- Microbial Inoculum: Use active, mature compost (e.g., from an industrial composting facility, sieved <10mm).
- Basal Medium: Prepare a mineral salts medium with ample nitrogen and phosphorus.
- Test Setup: In respirometric flasks, combine:
- Positive Control (PC): Basal medium + inoculum + certified reference material (cellulose powder).
- Negative Control (NC): Basal medium + inoculum + sodium azide (inhibitor).
- Test Series (T1-T4): Basal medium + inoculum + polymer leachate at concentrations of 10%, 25%, 50%, and 100% (v/v).
- Viability Control (VC): Basal medium + inoculum + leachate (any conc.) + a readily degradable carbon source (glucose).
- Incubation & Measurement: Incubate at 58°C (composting temperature). Monitor CO₂ production via titration, infrared detection, or pressure sensors daily for 5-7 days.
- Data Interpretation: Calculate the percentage of inhibition (I%) relative to the positive control. I% = [1 - (CO₂(Test) - CO₂(NC)) / (CO₂(PC) - CO₂(NC))] * 100 Inhibition is significant if I% > 25% in T1 (10% leachate). A recovery in VC indicates metabolic inhibition rather than biocidal activity.
Protocol 3.2: Molecular Analysis of Consortium Shifts
To confirm inhibition and identify sensitive taxa, perform 16S rRNA gene amplicon sequencing.
- Sampling: At the endpoint of Protocol 3.1, collect biomass from PC and inhibited test vessels.
- DNA Extraction: Use a commercial soil DNA extraction kit with mechanical lysis.
- Sequencing: Amplify the V3-V4 region of the 16S rRNA gene (primers 341F/806R) and sequence on an Illumina MiSeq platform (2x300 bp).
- Bioinformatics: Process sequences through QIIME2 or Mothur. Compare alpha-diversity (Shannon index) and beta-diversity (Weighted UniFrac) between PC and test samples. A significant drop in diversity and shift in community structure indicates selective inhibition.
Data Presentation
Table 1: Example Inhibition Data for a PLA Biopolymer with Residual Lactide Monomer
| Leachate Concentration (%) | Cumulative CO₂ (mg) at Day 7 | Inhibition (%) (I%) | Community Shannon Index |
|---|---|---|---|
| Positive Control (Cellulose) | 455 ± 12 | 0 (Ref) | 5.8 ± 0.2 |
| Negative Control (Azide) | 28 ± 4 | 100 | N/A |
| Test: 10% Leachate | 430 ± 15 | 5.5 | 5.6 ± 0.3 |
| Test: 25% Leachate | 310 ± 20 | 31.8* | 4.1 ± 0.4* |
| Test: 50% Leachate | 155 ± 10 | 66.0* | 3.5 ± 0.3* |
| Viability Control | 448 ± 18 | 1.5 | N/A |
*Indicates significant inhibition (I% > 25%, p < 0.05).
Table 2: Key Research Reagent Solutions & Materials
| Item & Example Product | Function in Inhibition Studies |
|---|---|
| Cellulose Microcrystalline (e.g., Avicel PH-105) | Positive control reference material; establishes baseline microbial activity in the specific inoculum. |
| Sodium Azide (NaN₃) | Negative control inhibitor; halts aerobic microbial respiration, defining the abiotic background. |
| Mineral Salts Medium (ISO 14855) | Provides essential nutrients (N, P, K, trace metals) to prevent limitation, ensuring only test article components cause inhibition. |
| Commercial Compost DNA Kit (e.g., DNeasy PowerSoil Pro) | Standardized, efficient extraction of high-quality microbial genomic DNA from complex organic matrices. |
| Universal 16S rRNA Primers (341F/806R) | Amplifies a conserved yet variable region for profiling bacterial/archaeal community composition. |
| Resazurin Sodium Salt | Redox indicator for quick viability assays; color change from blue to pink indicates active respiration. |
| Poly-β-Hydroxybutyrate (PHB) Powder | Alternative positive control; a pure, readily biodegradable biopolymer to cross-validate results. |
Visualization of Workflows & Pathways
Diagram 1: Tiered inhibition screening workflow.
Diagram 2: Primary biochemical pathways of microbial inhibition.
Mitigation & Best Practices
To prevent skewed data:
- Pre-Test Purification: Pre-wash or dialyze test materials to remove leachable inhibitors prior to the main test.
- Dose-Response Assessment: Always include a range of test material concentrations to identify a non-inhibitory level, as per OECD guidelines.
- Community Profiling: Use molecular tools (Protocol 3.2) not just for confirmation, but proactively to select robust, diverse inocula resistant to common inhibitors.
- Material Characterization: Fully characterize the test article via GC-MS (for residual monomers), ICP-MS (for heavy metals), and HPLC (for additive content) before biodegradation testing. Correlation of inhibition with a specific chemical is paramount.
By integrating these protocols and checks into the research framework for biopolymer standards, scientists can differentiate true material recalcitrance from methodological artifact, driving the development of genuinely biodegradable and compostable materials.
The reliable assessment of biopolymer biodegradability and compostability, as per standards such as ISO 14855 (controlled composting) and ASTM D5338, hinges on the precise maintenance of key environmental parameters. Moisture content and pH are two interdependent factors that critically influence microbial metabolism, enzyme activity, and ultimately, the rate and extent of polymer breakdown. This guide details the scientific and technical principles for managing these variables in both active composting facilities and laboratory-simulated environments, providing a foundation for reproducible research in biopolymer characterization.
The Interdependence of Moisture and pH
The Role of Moisture
Moisture acts as a solvent for microbial nutrients and metabolites, governing oxygen diffusion and the physical structure of the compost matrix. Suboptimal levels directly stall biodegradation.
- Low Moisture (<40%): Restricts microbial mobility, limits enzymatic hydrolysis, and reduces metabolic rates.
- High Moisture (>65%): Fills pore spaces, creating anaerobic zones, reducing heat retention, and promoting acidogenic pathways that lower pH.
The Role of pH
pH dictates the enzymatic efficiency and community structure of the microbial consortia responsible for degradation. Most composting microbes (bacteria, actinomycetes) thrive in neutral to slightly alkaline conditions.
- Low pH (<6.0): Favors acid-tolerant fungi over bacteria, slows lignocellulosic breakdown, and can indicate process failure (souring).
- High pH (>9.0): Can inhibit mesophilic microorganisms and indicate excessive ammonia volatilization.
Optimal ranges are defined by international standards for biodegradation testing. The following table consolidates target values and their impacts.
Table 1: Optimal Ranges and Impacts of Moisture and pH in Compost Environments
| Parameter | Optimal Range (Aerobic Composting) | Standard Reference | Consequence of Deviation |
|---|---|---|---|
| Moisture Content | 50% - 60% (w/w) | ISO 14855-1, ASTM D5338 | <40%: Microbial activity inhibited. >65%: Anaerobiosis, odor, heat loss. |
| pH | 6.5 - 8.5 | ISO 14855-2, EN 13432 | <6.0: Process inhibition, fungal dominance. >9.0: Ammonia loss, microbial toxicity. |
| C:N Ratio | 25:1 - 30:1 | General Composting Principle | Influences microbial growth rates and nitrogen availability, indirectly affecting pH. |
| Oxygen Concentration | >10% (headspace) | ASTM D5338 | Low O₂ leads to anaerobic conditions, acid production, and pH drop. |
Experimental Protocols for Monitoring and Control
Protocol A: Gravimetric Moisture Determination
Principle: Direct measurement of weight loss upon drying. Method:
- Weigh an empty, dry moisture dish (W₁).
- Add approximately 10g of fresh compost sample, spread evenly. Weigh dish + sample (W₂).
- Dry in an oven at 105°C for 24 hours or until constant weight.
- Place dish in a desiccator to cool, then weigh (W₃).
- Calculation: Moisture Content (%) = [(W₂ - W₃) / (W₂ - W₁)] * 100
Protocol B: In-situ pH Measurement in Compost/Simulated Media
Principle: Potentiometric measurement using a calibrated pH electrode. Method:
- Sample Preparation: Mix 10g of compost with 25mL of deionized water (1:2.5 w/v slurry). Shake vigorously for 60 seconds.
- Calibration: Calibrate a robust combination pH electrode using standard buffers at pH 4.01, 7.00, and 10.01.
- Measurement: Immerse the calibrated electrode in the supernatant of the settled slurry. Record the stable reading.
- Cleaning: Rinse electrode thoroughly with DI water between samples to prevent cross-contamination.
Protocol C: Moisture Adjustment in Simulated Reactors
Principle: Incremental addition of water or dry bulking agent to achieve target moisture. Method:
- Determine initial moisture content (Protocol A) and total mass (Mₜ) of the compost matrix in the reactor.
- Calculate target dry mass (DM): DM = Mₜ / (1 + (Target Moisture%/100)).
- Calculate required water addition: Water (g) = (DM * (Target Moisture%/100)) - Current Water Mass.
- Add calculated water incrementally with thorough mixing. Re-measure moisture after 2-4 hours of equilibration.
Visualizing the Moisture-pH-Biodegradation Relationship
Title: Moisture Impact on pH and Biodegradation Pathways
Title: Moisture & pH Maintenance Workflow for Biodegradation Tests
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Reagents and Materials for Compost Parameter Management
| Item | Function & Rationale |
|---|---|
| Forced-Air Oven (105°C) | Precise gravimetric determination of moisture content via dry-weight loss. |
| Analytical Balance (±0.01g) | Accurate weighing of samples, amendments, and test materials. |
| Robust Combination pH Electrode | Withstand abrasive compost slurry. Essential for in-situ pH tracking. |
| pH Buffer Solutions (4.01, 7.00, 10.01) | Mandatory for three-point calibration of pH meter for accuracy across range. |
| Calcium Carbonate (CaCO₃, Powder) | Common pH buffer to counteract acidification in simulated compost environments. |
| Ammonium Sulfate ((NH₄)₂SO₄) | Nitrogen source used to adjust the C:N ratio, which indirectly influences pH stability. |
| Dry, Mature Compost or Wood Chips | Used as a bulking agent to reduce moisture content and improve porosity/aeration. |
| Deionized Water | For preparing sample slurries for pH and for moisture adjustment; prevents ionic interference. |
| Insulated Composting Reactors | Lab-scale vessels (e.g., 2-10L) with air supply and gas traps for simulated environments per ISO 14855. |
| Data Logger with Probes | For continuous monitoring of temperature and humidity within reactor headspace. |
Within the rigorous framework of biopolymer biodegradability and compostability standards research, experimental data seldom conforms to idealized first-order kinetics. The real-world interpretation of biodegradation curves—specifically lag phases, plateauing, and incomplete mineralization—is critical for accurate material classification under standards such as ISO 14855, ASTM D6400, and EN 13432. For researchers and pharmaceutical development professionals utilizing biodegradable polymer matrices, these non-ideal phases reveal essential information about microbial inoculum vitality, substrate bioavailability, and potential inhibitory effects.
Quantitative Analysis of Non-Ideal Biodegradation Phases
Table 1: Common Non-Ideal Curve Phenomena in Standardized Tests
| Phenomenon | Typical Duration | Potential Causes | Impact on Final Mineralization (%) |
|---|---|---|---|
| Extended Lag Phase | >10% of total test duration | Microbial acclimation, lack of specific degraders, antimicrobial additives. | May delay but not necessarily reduce final extent. |
| Premature Plateau | Early stabilization of CO₂ evolution | Nutrient limitation (N, P), toxicity from intermediates, polymer crystallinity. | Directly reduces final biodegradation percentage. |
| Incomplete Mineralization | N/A (final measurement) | Presence of non-biodegradable components, synthesis impurities, toxic monomer residues. | Final plateau below required threshold (e.g., <90% for compostability). |
| Two-Phase Mineralization | Biphasic curve pattern | Degradation of labile additives first, then backbone; or sequential colonization by different consortia. | Can achieve high mineralization but with complex kinetics. |
Table 2: Regulatory Thresholds and Tolerances for Non-Ideality
| Standard | Maximum Lag Phase (Days) Considered Normal | Minimum Mineralization % for "Biodegradable" | Minimum Mineralization % for "Compostable" |
|---|---|---|---|
| ISO 14855-1 (Controlled Composting) | 10-15 | Not specified (kinetic parameter) | 90% (absolute, vs. positive control) |
| ASTM D5338 | 10 | 70% (relative to theoretical CO₂) | 90% (relative to theoretical CO₂) |
| OECD 301B | Up to 28 (10-day window not always required) | 60% (ThOD) within 28-day window | N/A |
Experimental Protocols for Diagnosing Causes
Protocol 1: Distinguishing between Inoculum vs. Substrate-Limited Lag Phases
- Objective: Determine if a prolonged lag phase is due to inadequate microbial inoculum or polymer characteristics.
- Method:
- Set up standard biodegradation reactors (e.g., respirometric units) per ISO 14855-1.
- Prepare two additional test sets:
- Set A (Inoculum Enrichment): Pre-incubate the compost inoculum with a small, soluble amount of the test polymer (or structural analog) for 7 days prior to the main test.
- Set B (Bioavailability Check): Incorporate a finely milled (<50 µm) version of the polymer alongside the standard form.
- Run all sets simultaneously under identical conditions.
- Analysis: A significantly reduced lag in Set A indicates an inoculum limitation. A reduced lag in Set B suggests bioavailability (surface area, crystallinity) is the primary cause.
Protocol 2: Investigating Plateauing via Nutrient Supplementation & Community Analysis
- Objective: Identify if a premature plateau is due to nutrient depletion or microbial community shifts.
- Method:
- Monitor standard test until the CO₂ evolution rate decreases significantly (<1% per day).
- Aseptically open replicate reactors at the plateau point.
- For half, supplement with a sterile, aqueous solution of essential nutrients (e.g., NH₄Cl, KH₂PO₄).
- For the other half, extract genomic DNA from the compost matrix for 16S rRNA gene amplicon sequencing to compare community structure to pre-plateau and positive control samples.
- Reseal and continue monitoring CO₂ evolution.
- Analysis: Resumption of mineralization post-nutrient addition indicates nutrient limitation. Community analysis may reveal a decline or shift in key degraders, suggesting toxicity or ecological succession issues.
Visualizing Diagnostic Pathways and Workflows
Title: Diagnostic Pathway for Non-Ideal Biodegradation Curves
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Investigating Non-Ideal Mineralization
| Item / Reagent | Function & Rationale |
|---|---|
| Activated Mature Compost (INOCULUM) | Standardized microbial source per ISO 14855. Variability here is a major cause of inter-lab discrepancy. |
| Cellulose Microcrystalline (POSITIVE CONTROL) | Validates inoculum activity. Any non-ideality in this curve invalidates the test run. |
| Polyethylene Powder (NEGATIVE CONTROL) | Confirms absence of abiotic CO₂ release and baseline microbial respiration. |
| Triphenyl Tetrazolium Chloride (TTC) / Dehydrogenase Assay Kit | Cell viability stain to quantify active microbial biomass, diagnosing inhibitory/toxic effects causing lags/plateaus. |
| Nutrient Stock Solutions (N, P, Trace Metals) | For supplementation experiments to diagnose nutrient-limited plateaus. Must be sterile and non-inhibitory. |
| DNA Extraction Kit (for Soil/Compost) | Enables molecular microbial community analysis (qPCR, 16S sequencing) to track degrader populations. |
| Headspace CO₂ Analyzer (e.g., GC-TCD, NDIR) | Essential for high-frequency, precise measurement of mineralization progress without disturbing the system. |
| Polymer Milling/Cryogrinding Apparatus | To modify particle size and crystallinity for bioavailability studies. |
The accurate characterization of biopolymer biodegradability and compostability under standardized test methods (e.g., ISO 14855, ASTM D6400) is fundamentally dependent on the analysis of a representative material sample. The inherent heterogeneity of advanced material formulations—including polymer blends, block copolymers, and biocomposites reinforced with natural fibers or fillers—poses a significant challenge. Non-representative sampling can lead to erroneous data on disintegration rates, microbial assimilation, and ultimate biodegradation, compromising the validity of certification for industrial compostability or environmental fate. This guide details rigorous methodologies to ensure statistical confidence in sampling, directly supporting reproducible and reliable research within biopolymer standards development.
Quantifying Heterogeneity and Determining Sample Size
The first step is a preliminary assessment of heterogeneity to define a statistically sound sampling plan. Key metrics include the proportion of components and their spatial distribution variance.
Table 1: Common Heterogeneity Metrics and Target Variances for Biopolymers
| Metric | Formula/Description | Target for Representative Sampling (Typical) |
|---|---|---|
| Compositional Variance (σ²c) | Variance in % weight of primary component across initial bulk scans (e.g., via handheld FTIR or XRF). | σ²c < 2.5 for blends; < 1.0 for copolymers |
| Particle Size Distribution (PSD) | Span = (D90 - D10) / D50, for granulated or powdered materials. | Span < 2.0 for consistent digestion |
| Spatial Autocorrelation Range | Distance over which material properties are correlated (from variogram analysis). | Sampling interval must exceed this range. |
The minimum number of incremental samples (n) to form a composite can be estimated using Gy's sampling theory, adapted for polymers:
n ≥ ( σ²ᵢ * t² ) / ( E² - (σ²ₐ / m) )
Where σ²ᵢ is ingredient variance, t is Student's t-value for confidence level, E is target sampling error, σ²ₐ is analytical variance, and m is replicate analyses.
Core Sampling Protocols & Experimental Methodologies
Protocol 2.1: Incremental Sampling for Bulk Powdered Blends (e.g., PLA-Starch Blends)
Objective: To collect a representative composite sample from a lot of powdered or granulated blend. Materials: Sampling thief (riffle divider), stainless steel scoops, composite container, rotary cup mill for size reduction. Procedure:
- Stratification: Divide the lot (e.g., production batch) into imaginary 3D strata.
- Random Increment Selection: Using a random number generator, select at least 30 increments from across all strata.
- Extraction: Use a sampling thief inserted at multiple depths to extract each increment.
- Compositing & Reduction: Combine all increments. Pass through a rotary riffle divider at least 8 times to homogenize and reduce mass.
- Final Sample Preparation: Grind a subset of the reduced composite in a cryo-mill (if Tg is below ambient) to achieve a defined PSD (e.g., < 1mm) for biodegradation testing.
Protocol 2.2: Systematic Random Sampling for Composite Film or Sheet
Objective: To sample from a roll or batch of film/ sheet with potential gradient or layered heterogeneity. Materials: Precision cutter (cork borer, laser cutter), template grid, marking tool. Procedure:
- Grid Definition: Overlay a Cartesian grid on the material sheet. For a roll, unroll and flatten a defined length.
- Random Start, Systematic Selection: Choose a random start point within the first grid cell. Then sample at regular intervals (every k-th cell) across the entire area.
- Core Extraction: Use a clean, sharp cutter to extract circular or square specimens from selected cells. For laminates, ensure full-thickness cores.
- Pooling: For chemical analysis (e.g., GPC, NMR), cut each core into small pieces and randomly select fragments from each to form the analytical sample.
Protocol 2.3: Cross-Sectional Micro-Sampling for Copolymer Fibers or Filaments
Objective: To analyze composition along and across copolymer fibers that may have core-shell or gradient morphology. Materials: Microtome (cryogenic if needed), fine-forceps, solvent wells, micro-balance. Procedure:
- Bundle Preparation: Align and epoxy a fiber bundle into a mounting block. Cure fully.
- Cross-Sectioning: Using a microtome, serially section the block perpendicular to fiber axis. Collect sections at defined intervals (e.g., every 10µm).
- Longitudinal Sectioning: Section a separate bundle parallel to the fiber axis to probe core-shell structure.
- Micro-analysis: Pool sections from equivalent depths for FTIR-ATR, DSC, or solvent extraction to determine localized composition.
Analytical Verification of Homogeneity
Post-sampling, verify representativeness before biodegradation experiments.
Table 2: Analytical Methods for Homogeneity Verification
| Method | Measured Parameter | Sample Prep | Acceptability Criterion |
|---|---|---|---|
| TGA-FTIR | Deconvolution of decomposition steps for blend components. | ~5 mg powder from 10+ sub-samples. | Coefficient of Variation (CV) of component % < 5%. |
| µ-XRF Mapping | Elemental distribution (e.g., Ca in filler, Si in coatings). | Film cross-section or flat sheet. | Relative Standard Deviation (RSD) of map intensity < 10%. |
| DSC (First Heat) | Enthalpy of melting (ΔHm) for crystalline phases. | 3-5 mg sealed pan from 8+ random points. | RSD of ΔHm < 7%. |
| Py-GC/MS | Characteristic pyrolysis products ratio (e.g., lactide/starch). | < 0.5 mg from multiple cores. | RSD of marker ratio < 15%. |
Integration with Biodegradation Testing Workflow
A standardized workflow from sampling to data interpretation is critical.
Title: Workflow for Representative Biodegradation Testing
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Representative Sampling & Analysis
| Item | Function in Sampling/Analysis | Key Consideration for Biopolymers |
|---|---|---|
| Cryogenic Mill (e.g., SPEX SamplePrep) | Homogenizes brittle, temperature-sensitive materials without altering chemistry. | Prevents thermal degradation of PLA, PHA during grinding; use liquid N₂. |
| Rotary Riffle Divider | Provides unbiased, geometrical division of particulate samples. | Essential for starch-PBAT powder blends; ensures statistical reduction. |
| Microtome with Cryo-Chamber | Produces thin, consistent cross-sections of fibers and films. | Necessary for Tg below RT (e.g., some PHBs); prevents smearing. |
| Stainless Steel Sampling Thief | Retrieves core increments from deep within powder containers. | Must be solvent-cleaned to avoid contamination from prior samples. |
| ICP-MS Calibration Standards | Quantifies trace metal catalysts (Sn, Zn, Al) affecting biodegradation. | High-purity standards required to trace residual catalyst variance. |
| Deuterated Solvents for NMR (e.g., CDCl₃, DMSO-d₆) | Dissolves polymers for quantitative ¹³C NMR to assess copolymer sequencing. | Must be anhydrous to prevent hydrolysis during analysis. |
| NIST-Traceable Reference Materials (e.g., PE, Cellulose) | Validates TGA, DSC instrument response and analytical precision. | Used in parallel with test samples to calibrate decomposition metrics. |
| Sterile Inoculum for Biodegradation | Standardized microbial community for compostability tests. | Must be verified for activity (e.g., using cellulose positive control). |
For research aimed at establishing robust biodegradability and compostability standards, the precision of the final analytical data is inextricably linked to the representativeness of the initial sample. Adherence to statistically designed sampling plans, followed by rigorous homogenization and verification, transforms a heterogeneous material into a reliable experimental input. This practice is non-negotiable for generating comparable, defensible data that can accurately classify the environmental performance of next-generation biopolymers.
Within the broader thesis on Biopolymer biodegradability and compostability standards research, the validation of testing methodologies is paramount. Accelerated testing protocols are indispensable tools for predicting the long-term environmental fate and functional lifetime of biopolymer-based materials, from packaging to biomedical implants. However, the extrapolation of accelerated data to real-world scenarios necessitates a critical understanding of the kinetic relationships and degradation mechanisms. This guide examines the principles of accelerated testing for biopolymer degradation, its inherent limitations, and the statistical and mechanistic frameworks required for robust correlation with real-time studies, thereby informing reliable standard development.
Core Principles and Kinetic Foundations
Accelerated degradation studies rely on the principle of stress testing, where elevated stress factors (e.g., temperature, humidity, oxidative load, enzymatic concentration) are applied to increase the rate of chemical or physical processes. The Arrhenius equation is the foundational model for temperature acceleration:
k = A exp(-Eₐ/RT)
Where:
- k = degradation rate constant
- A = pre-exponential factor
- Eₐ = activation energy (J/mol)
- R = universal gas constant (8.314 J/mol·K)
- T = absolute temperature (K)
For biopolymers, degradation can follow various pathways (hydrolytic, enzymatic, oxidative), each with its own activation energy. Accurate extrapolation requires confirming that the acceleration factor does not alter the fundamental degradation mechanism.
Benefits of Accelerated Testing
The primary benefits are summarized in the table below.
Table 1: Key Benefits of Accelerated Testing in Biopolymer Research
| Benefit | Description | Impact on Biopolymer Research |
|---|---|---|
| Time Efficiency | Reduces test duration from years/months to weeks/months. | Enables rapid screening of novel polymer formulations and composites. |
| Cost Reduction | Lowers long-term facility and monitoring costs. | Makes material development cycles more economical and iterative. |
| Mechanistic Insight | High-stress conditions can isolate and amplify specific degradation pathways. | Helps identify rate-limiting steps in hydrolysis or enzymatic breakdown. |
| Quality Control | Facilitates batch-to-batch consistency checks and shelf-life predictions. | Critical for commercial production of biomedical devices (e.g., absorbable sutures, scaffolds). |
| Standardization | Provides a controlled, reproducible framework for comparative material assessment. | Forms the basis for ISO/ASTM compostability and biodegradability standards. |
Limitations and Critical Challenges
Accelerated methods introduce risks that can invalidate correlation with real-time data.
Table 2: Key Limitations and Challenges of Accelerated Testing
| Limitation | Technical Description | Consequence for Biopolymers |
|---|---|---|
| Mechanism Shift | Elevated stress (e.g., extreme pH, temperature) may initiate secondary reactions (e.g., cross-linking vs. chain scission) not seen under ambient conditions. | Predictions of degradation products and mechanical loss become inaccurate. |
| Product Diffusion Effects | Accelerated breakdown can saturate the local environment with oligomers, altering local pH and enzymatic activity, a phenomenon not observed in slow, real-time degradation. | Over- or under-estimation of biodegradation rates in compost or marine environments. |
| Neglected Low-Temperature Phenomena | Processes like microbial population succession in compost or crystalline reorganization in polymers may not be activated at high temperatures. | Fails to predict long-term behavior in real-world disposal systems. |
| Activation Energy Uncertainty | Using an incorrect Eₐ value for extrapolation leads to large errors. Eₐ must be empirically derived for each material and pathway. | A 10 kJ/mol error in Eₐ can lead to a ~2-3x error in predicted lifetime at 25°C. |
| Statistical Uncertainty | Limited sample size at high acceleration factors amplifies the confidence interval of predictions. | Requires sophisticated reliability statistics (e.g., Weibull analysis) for safe extrapolation. |
Methodological Framework for Correlation
Correlation is not assumed; it must be demonstrated through a structured protocol.
Experimental Protocol 1: Establishing a Predictive Arrhenius Model
- Material Preparation: Prepare identical specimens (e.g., 10mm x 10mm films, powder) from the biopolymer batch. Characterize initial molecular weight (GPC), crystallinity (DSC), and mechanical properties.
- Accelerated Conditions: Subject specimens to hydrolytic degradation in pH 7.4 phosphate buffer at a minimum of three elevated temperatures (e.g., 37°C, 50°C, 70°C). Use a minimum of n=5 replicates per time point.
- Real-Time Condition: Run a parallel, real-time study at the target temperature (e.g., 25°C).
- Monitoring: At predetermined intervals, remove samples. Measure molecular weight (Mₙ) and mass loss. Plot Ln(Mₙ) vs. time to determine the rate constant (k) at each temperature.
- Arrhenius Plot: Plot Ln(k) vs. 1/T. Perform linear regression to determine the slope (-Eₐ/R) and intercept (Ln(A)).
- Prediction & Validation: Use the Arrhenius equation to predict the degradation rate at 25°C. Compare the predicted degradation profile (e.g., time to 50% Mₙ loss) with the ongoing data from the real-time study. Statistical overlap (e.g., via confidence intervals) validates the model.
Experimental Protocol 2: Mechanistic Consistency Check via FTIR Spectroscopy
- Sampling: Collect degraded samples from each condition in Protocol 1 at equivalent stages of degradation (e.g., at 20%, 50% mass loss).
- Analysis: Perform FTIR spectroscopy in ATR mode. Focus on characteristic bands (e.g., ester C=O stretch ~1750 cm⁻¹, hydroxyl O-H stretch ~3400 cm⁻¹).
- Comparison: Use difference spectroscopy or peak deconvolution to track the formation of specific functional groups (e.g., carboxylic acids). A consistent spectral evolution across all temperatures indicates a constant mechanism.
Flowchart: Correlation Validation Workflow for Degradation Studies
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Biopolymer Degradation Studies
| Item / Reagent | Function & Technical Rationale |
|---|---|
| Phosphate Buffered Saline (PBS), pH 7.4 | Standardized hydrolytic medium for simulating physiological or neutral environmental conditions. Ionic strength affects degradation kinetics. |
| Controlled-Compost Inoculum | Standardized microbial community (per ISO 14855) for assessing biodegradation under aerobic composting conditions, crucial for compostability claims. |
| Specific Enzymes (e.g., Proteinase K, Lipase, Cellulase) | Used to isolate and study enzymatic degradation pathways specific to polymer bonds (amide, ester, glycosidic), providing mechanistic clarity. |
| Molecular Weight Standards (for GPC/SEC) | Narrow dispersity polystyrene or polymethylmethacrylate standards for calibrating Gel Permeation Chromatography, essential for tracking chain scission. |
| Deuterated Solvents (e.g., D₂O, CDCl₃) | Required for NMR spectroscopy to analyze degradation products and confirm chemical structure changes without proton interference. |
| Thermogravimetric Analysis (TGA) Calibration Standards | Certified metals (e.g., Curie point standards: Alumel, Nickel) to validate temperature accuracy and mass loss measurements during thermal degradation studies. |
| FTIR ATR Crystal Cleaning Kit | Solvents and polishing materials for maintaining the crystal (ZnSe, Diamond) to ensure consistent, high-quality spectral data for mechanism verification. |
Data Synthesis and Correlation Metrics
Quantitative correlation requires robust metrics beyond simple rate comparisons.
Table 4: Key Metrics for Evaluating Correlation Between Accelerated and Real-Time Data
| Metric | Calculation / Method | Interpretation |
|---|---|---|
| Time-Transformation Factor (TTF) | TTF = treal / tacc for equivalent % property loss. | A constant TTF across multiple degradation levels (e.g., 10%, 50%, 80% loss) supports valid acceleration. |
| Mechanistic Correlation Coefficient | Statistical comparison (e.g., Pearson's r) of degradation product ratios (from HPLC/GC-MS) between conditions. | An r > 0.9 indicates high mechanistic fidelity. |
| Prediction Interval Accuracy | Compare the 95% prediction interval from the accelerated model with the actual real-time data points. | Real-time data should fall within the prediction interval for the model to be considered valid. |
| Shape Parameter (β) Consistency (Weibull Analysis) | Fit degradation data (e.g., tensile strength retention) to a Weibull distribution. A constant β indicates consistent failure mode. | A shift in β between accelerated and real conditions signals a change in the dominant failure mechanism. |
In biopolymer research for standards development, accelerated testing is a powerful but double-edged tool. Its benefits of speed and cost can only be leveraged when its limitations are rigorously addressed. Valid correlation is not a given; it is an experimental result achieved through parallel mechanistic studies, multi-stress-factor kinetic modeling, and continuous validation against real-time data. A scientifically robust standard must, therefore, prescribe not just the accelerated test conditions but also the mandatory mechanistic checks and correlation requirements needed to ensure predictions of biodegradability and compostability are both accurate and environmentally relevant.
Benchmarking & Compliance: Comparative Analysis of Certification Schemes and Regulatory Implications
Within the framework of biopolymer biodegradability and compostability standards research, a critical analysis of the three predominant international standards—ASTM D6400, EN 13432, and ISO 17088—is essential. These standards establish the pass/fail criteria that determine if a material can be certified as industrially compostable. While harmonized in core principles, differences in testing stringency, specific thresholds, and methodological details create a complex landscape for researchers and product developers. This guide provides a technical deconstruction and comparison of these pivotal documents.
Core Principles and Scope
All three standards define the requirements for plastics designed to be composted in municipal and industrial aerobic composting facilities. They are based on a four-pillar framework: 1) Biodegradation, 2) Disintegration, 3) Lack of Ecotoxicity, and 4) Control of Heavy Metals/Substances. The ultimate goal is to ensure that certified materials will fully break down in an industrial composting process without leaving harmful residues.
Quantitative Pass/Fail Criteria Comparison
The following tables summarize the key quantitative requirements. Note that EN 13432 and ISO 17088 are closely aligned, while ASTM D6400 shows some variations.
Table 1: Core Performance Requirements
| Criterion | ASTM D6400 | EN 13432 | ISO 17088 | Common Test Method |
|---|---|---|---|---|
| Biodegradation (Mineralization) | ≥90% absolute or ≥90% of reference material over ≤180 days | ≥90% absolute degradation over ≤6 months | ≥90% absolute degradation over ≤6 months | ISO 14855 (controlled composting) |
| Disintegration | ≤12 weeks; ≤10% residue on 2mm sieve | ≤12 weeks; ≤10% dry mass residue on 2mm sieve | ≤12 weeks; ≤10% dry mass residue on 2mm sieve | ISO 16929 or ISO 20200 (pilot-scale) |
| Ecotoxicity (Plant Growth) | ≥90% of control in germination & biomass tests | ≥90% of control in germination & plant biomass | ≥90% of control in germination & plant biomass | OECD 208 (modified) with compost post-test |
| Heavy Metals | Defines specific limits (e.g., Cd: 0.5 mg/kg, Pb: 50 mg/kg) | Must meet national limits (e.g., EU Directive 2000/76/EC) | Must meet limits of the country where compost is marketed | Chemical analysis (ICP-MS, AAS) |
Table 2: Additional Chemical & Substance Requirements
| Parameter | ASTM D6400 | EN 13432 | ISO 17088 |
|---|---|---|---|
| Fluorine Content | ≤100 ppm (Proposed) | ≤100 ppm | Must be declared; limits as per national regulation. |
| Volatile Solids (Organic Content) | ≥50% | ≥50% | ≥50% (for biodegradation calculation) |
| pH | Not directly specified for final compost | Within defined limits for ecotoxicity test | Within defined limits for ecotoxicity test |
| Salinity | Not directly specified | Within defined limits for ecotoxicity test | Within defined limits for ecotoxicity test |
Detailed Experimental Protocols
Biodegradation Test (ISO 14855-1/2)
Objective: To determine the ultimate aerobic biodegradability of plastic materials under controlled composting conditions by measuring evolved CO₂. Methodology:
- Inoculum: Mature, stable compost derived from the organic fraction of municipal solid waste.
- Test Material: Prepared to particles ≤2mm. A cellulose reference material is run in parallel.
- Reactors: Vessels containing a mixture of test material (or reference) and inoculum in a solid, porous matrix. CO₂-free air is passed through.
- Incubation: At 58°C ± 2°C (thermophilic conditions) for up to 180 days.
- Measurement: The CO₂ evolved from each vessel is trapped in a solution of NaOH or Ba(OH)₂ and quantified by titration. Alternatively, a direct gas analysis system (e.g., GC, IR) is used in ISO 14855-2.
- Calculation: Biodegradation (%) = [(CO₂)test – (CO₂)blank] / (ThCO₂) * 100, where ThCO₂ is the theoretical amount of CO₂ produced from complete oxidation of the test material.
Disintegration Test (ISO 16929)
Objective: To determine the degree of disintegration of plastic materials under simulated industrial composting conditions on a pilot scale. Methodology:
- Composting Bin: A pilot-scale composting reactor (e.g., 100-200 L) with forced aeration and temperature control.
- Test Material: Specimens of the finished product are placed in synthetic mesh bags.
- Process: The material is composted with fresh organic waste (bio-waste) under controlled conditions (temperature profile: thermophilic phase for several weeks, followed by curing). The compost is regularly turned.
- Sampling & Analysis: After a defined period (e.g., 12 weeks), the mesh bags are retrieved. The contents are washed and sieved over a 2.0 mm sieve. The residue >2.0 mm is dried and weighed.
- Calculation: Disintegration (%) = [(Initial dry mass – Final dry mass >2mm) / Initial dry mass] * 100.
Ecotoxicity Test (Modified OECD Guideline 208)
Objective: To assess the quality of the resulting compost by evaluating its effect on plant growth. Methodology:
- Test Compost: Compost from the disintegration test is used.
- Control Compost: Compost from a blank run (without test material).
- Test Plants: Two higher plant species (e.g., barley (Hordeum vulgare), cress (Lepidium sativum), or ryegrass (Lolium perenne)) are selected.
- Procedure: Seeds are planted in pots containing 100% test compost or 100% control compost. Plants are grown under controlled light and temperature for a period (e.g., 14-21 days).
- Endpoints: Germination percentage and total plant biomass (dry weight) are measured.
- Criterion: The biomass and germination in the test compost must be ≥90% of the control.
Visualizing the Standard Compliance Workflow
Title: Compostability Certification Testing Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
| Item | Function in Testing | Typical Specification / Example |
|---|---|---|
| Mature Compost Inoculum | Source of microorganisms for biodegradation & disintegration tests. Must be stable and active. | VS >30%; pH 7-9; from biowaste. |
| Microcrystalline Cellulose | Positive control reference material for biodegradation tests. | Particle size <20 µm, >99% purity. |
| Barium Hydroxide / Sodium Hydroxide Traps | For absorption and quantification of CO₂ in biodegradation tests (ISO 14855-1). | 0.05M-0.1M Ba(OH)₂, standardized. |
| Synthetic Compost Feedstock | Defined organic waste mixture for disintegration tests (ISO 20200). | Recipe: 40% sawdust, 30% rabbit feed, 10% ripe compost, etc. |
| Test Plant Seeds (Cress, Barley) | For ecotoxicity (phytotoxicity) assays. Must have high germination rate. | Lepidium sativum, Hordeum vulgare. |
| ICP-MS Calibration Standards | For precise quantification of regulated heavy metals (Cd, Pb, Hg, etc.). | Multi-element standard solutions. |
| Ion Chromatography Standards | For analysis of fluoride and other anions if required. | Sodium fluoride standard. |
| 2.0 mm Test Sieve | For separation and quantification of disintegration residues. | Stainless steel, certified mesh size. |
| Gas Analysis System (IR/GC) | For direct measurement of CO₂ and O₂ in biodegradation tests (ISO 14855-2). | Continuous or intermittent flow systems. |
Within biopolymer research, the validation of biodegradability and compostability claims is paramount for scientific credibility and regulatory acceptance. Certification labels, such as OK compost INDUSTRIAL and the Seedling Logo, serve as critical third-party verification tools. This guide analyzes these labels from a research perspective, detailing their technical specifications, testing protocols, and implications for market access, framed within a thesis on biopolymer standards.
Key Certification Schemes: Technical Specifications & Data
Certification labels are awarded based on compliance with established standards, primarily the European Norm (EN) standards and ISO standards. The following table summarizes the core quantitative requirements for key certifications relevant to industrial and home compostability.
Table 1: Quantitative Requirements for Major Compostability Certifications
| Certification Label / Standard | Reference Standard | Required Biodegradation (Min.) | Required Disintegration (Max. Residue) | Ecotoxicity Requirement | Max. Regulated Metals | Key Application Scope |
|---|---|---|---|---|---|---|
| OK compost INDUSTRIAL | EN 13432 / ASTM D6400 | 90% (absolute) or 90% of reference in ≤ 180 days (58°C ±2°C) | <10% of original mass on 2mm sieve after 12 weeks | No adverse effects on compost quality | Meets EN 13432 limits | Industrial composting facilities |
| Seedling Logo (EU) | EN 13432 | 90% (absolute) or 90% of reference in ≤ 6 months | <10% of original mass on 2mm sieve after 12 weeks | Pass germination & plant growth tests | Meets EN 13432 limits | Industrial composting (packaging) |
| OK compost HOME | AS 5810 / NF T51-800 | 90% in ≤ 365 days (20-30°C) | <10% of original mass on 2mm sieve | No adverse effects on compost quality | Meets standard limits | Home composting conditions |
| ASTM D6400 | ASTM D6400 | 90% of carbon to CO₂ in ≤ 180 days (58°C) | <10% of original mass on 2mm sieve after 12 weeks | No negative impact on plants | Specified limits | Industrial composting (US focus) |
Data synthesized from TÜV AUSTRIA, European Bioplastics, and ASTM International standards documentation (2023-2024).
Core Experimental Protocols for Certification
The certification process relies on rigorous, standardized laboratory and pilot-scale tests. The following methodologies are foundational.
Protocol for Aerobic Biodegradation under Controlled Composting Conditions (EN 14046 / ISO 14855)
Objective: To determine the ultimate aerobic biodegradability of plastic materials under simulated industrial composting conditions. Materials:
- Inoculum: Mature, stable compost (particle size <10mm).
- Test Material: Pulverized polymer sample (particle size <250µm) with known carbon content.
- Positive Control: Microcrystalline cellulose.
- Bioreactors: Vessels with forced aeration and temperature control.
- CO₂ Trapping System: Such as NaOH solution baths. Procedure:
- Mix test material with inoculum in reactors. Maintain at 58°C ±2°C.
- Continuously aerate with CO₂-free, humidified air.
- Regularly titrate the NaOH traps to quantify evolved CO₂.
- Continue for a maximum of 6 months.
- Calculate the percentage biodegradation relative to the theoretical maximum CO₂ production from the sample. Data Analysis: Biodegradation percentage is plotted over time. The plateau phase must reach the required threshold (e.g., 90%).
Protocol for Disintegration Testing (EN 14045)
Objective: To assess the physical fragmentation of the material during composting. Materials:
- Test Material: Labeled samples (e.g., 20x20mm films).
- Inoculum: Active composting material.
- Test Containers: Perforated vessels (≈3L).
- Sieve: 2.0 mm mesh. Procedure:
- Place pre-weighed test samples within mesh bags and mix into inoculum in containers.
- Incubate under optimal composting conditions (58°C, controlled moisture) for 12 weeks.
- After incubation, recover the test material residues, wash, and dry.
- Sieve the residues over a 2.0 mm sieve. Weigh the material retained. Data Analysis: Calculate the percentage of original dry mass remaining as residue >2mm. Must be <10%.
Protocol for Ecotoxicity Testing (EN 13432)
Objective: To evaluate the impact of compost residues on plant growth. Materials:
- Test Compost: Final compost from the disintegration test.
- Control Compost: Compost from a blank run (without test material).
- Plant Species: Two higher plants (e.g., cress, barley).
- Growth Trays & Controlled Environment Chamber. Procedure:
- Mix test and control composts with a standard soil substrate in defined ratios.
- Sow seeds in the mixtures and grow under standardized conditions.
- Monitor and measure germination rate, biomass, and plant health over a set period (e.g., 14-21 days). Data Analysis: Compare biomass and germination percentage in test versus control. Inhibition must not be statistically significant.
Visualizing Certification Pathways & Workflows
Diagram 1: Certification Process Workflow (78 chars)
Diagram 2: Core Test Sequence for EN 13432 (67 chars)
The Scientist's Toolkit: Key Research Reagents & Materials
Table 2: Essential Research Reagents for Compostability Testing
| Item / Reagent | Function in Experimental Protocol | Technical Specification / Note |
|---|---|---|
| Mature Compost Inoculum | Provides active microbial community for biodegradation testing. Source-critical for reproducibility. | Particle size <10mm, stable pH, from industrial composting plant. Must be verified for activity. |
| Microcrystalline Cellulose (Avicel PH-105) | Positive control material with known, high biodegradability. | ≥97% alpha-cellulose, particle size ~20µm. Serves as carbon source reference. |
| Sodium Hydroxide (NaOH) Solution (0.05-0.5M) | Traps evolved CO₂ in biodegradation reactors for quantitative titration. | Must be CO₂-free, prepared with degassed deionized water. Standardized regularly. |
| Barium Chloride (BaCl₂) Solution | Used in titration to precipitate carbonate, allowing precise measurement of trapped CO₂. | Typically 1.0M solution. Critical for two-stage titration method (ISO 14855). |
| Standard Soil Substrate | Defined growth medium for ecotoxicity (plant germination and growth) tests. | Low nutrient, standardized composition (e.g., LUFA 2.2). Ensures test sensitivity. |
| Reference Seeds (Cress, Barley) | Biological indicators for compost ecotoxicity assessment. | Must be from certified, high-germination-rate batches (>90%). |
| Vermiculite/Perlite | Bulking agent in disintegration test vessels to maintain porosity and aerobic conditions. | Inert, sterile, consistent particle size. |
| Elemental Analysis Standards | For calibrating CHNS/O analyzers to determine carbon content of test materials. | Certified Reference Materials (CRMs) for accurate %C calculation. |
This whitepaper, framed within a broader thesis on biopolymer biodegradability and compostability standards research, investigates two critical environments not covered by industrial composting (ASTM D6400/ISO 17088): marine ecosystems and home composting systems. The persistence of plastic pollution necessitates validating material performance in these realistic, lower-management scenarios. This guide provides researchers and drug development professionals—particularly those exploring biodegradable polymer matrices for controlled release—with the technical frameworks for conducting and interpreting these specific validations.
ASTM D6691: Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment
Objective & Principle
This method determines the degree and rate of aerobic biodegradation of plastic materials when exposed to a natural seawater inoculum under controlled laboratory conditions. Biodegradation is measured by quantifying the amount of carbon dioxide (CO₂) produced over time compared to theoretical amounts.
Detailed Experimental Protocol
A. Apparatus & Setup:
- Bioreactors: Erlenmeyer flasks (1-2 L) or specialized respirometric vessels equipped with CO₂-trapping compartments.
- CO₂ Measurement System: Titration setup for alkaline traps, or preferably, Continuous CO₂ Evolution Monitoring System (e.g., GC, IR detectors).
- Environmental Chamber: Capable of maintaining temperature at 30±2°C, with agitation (e.g., shaker platform).
- Seawater Collection & Preparation: Natural seawater is filtered (e.g., through 0.45 µm filter) to remove large particulates while retaining microbial community. Must be used within 36 hours of collection.
B. Test Material Preparation:
- Prepare test material, positive control (e.g., cellulose powder), and negative control (none, or a non-biodegradable polymer like PE) as powders or small pieces (<250 µm thick, <20 mg weight).
- Weigh material to provide 100-200 mg of organic carbon per vessel.
- Pre-condition materials (optional but recommended) by soaking in sterile seawater for 24 hours.
C. Test Execution:
- Basal Medium Preparation: Add inorganic nutrients (NH₄Cl, K₂HPO₄, etc.) to filtered seawater to ensure nutrient availability does not limit biodegradation.
- Vessel Inoculation: To each vessel, add:
- 800-900 mL of prepared seawater inoculum.
- 100-200 mg carbon equivalent of test/control material.
- Ensure C:N:P ratio is at least 40:10:1.
- CO₂ Trapping: For static systems, connect vessel to a headspace purged with CO₂-free air, leading to a series of traps containing 0.1-0.5 N NaOH solution.
- Incubation: Incubate vessels in the dark at 30°C with continuous agitation for up to 180 days.
- Monitoring: At regular intervals (e.g., days 1, 3, 7, then weekly):
- Quantify CO₂ in traps via titration with HCl (BaCl₂ addition for carbonate precipitation) or record data from continuous monitor.
- Measure pH and O₂ concentration.
- Visually note changes to material.
D. Calculations:
% Biodegradation = [(CO₂)sample - (CO₂)blank] / ThCO₂ * 100
Where ThCO₂ is the theoretical amount of CO₂ produced if the test material is completely mineralized.
Key Data & Interpretation
Table 1: Example Marine Biodegradation Data (ASTM D6691) for Selected Materials
| Material Type | Thickness (µm) | Time to 10% Degradation (days) | Time to 90% Degradation (days) | Maximum Biodegradation (% of ThCO₂) at 180 days | Final Disintegration Observation |
|---|---|---|---|---|---|
| Microcrystalline Cellulose (Control) | Powder | 5 ± 2 | 28 ± 5 | 98 ± 3 | Complete |
| PHA (polyhydroxyalkanoate) Film | 50 | 15 ± 5 | 90 ± 15 | 95 ± 5 | Complete, fragmented |
| PLA (polylactic acid) Film | 100 | >180* | >180* | <5* | Intact |
| Starch-based Composite | 200 | 30 ± 10 | 120 ± 20 | 85 ± 10 | Partial fragmentation |
| LDPE (Negative Control) | 100 | >180* | >180* | <1* | Intact |
* No significant degradation observed within test period.
Pass/Fail Criterion: While D6691 is a measurement method, a common benchmark for "marine biodegradable" is >90% of the positive control's degradation rate or >90% absolute mineralization within 6 months.
Home Composting Performance Validation
Objective & Principle
There is no single global standard for home composting (e.g., AS 5810, NF T51-800, OK compost HOME). Validation typically assesses the disintegration and biodegradation of materials under simulated, mesophilic home compost conditions (lower, more variable temperatures than industrial composting).
Detailed Experimental Protocol (Simulated Home Compost)
A. Apparatus & Setup:
- Compost Vessels: Insulated containers (e.g., 10-50 L bins) that allow for passive aeration, or controlled reactors.
- Compost Matrix: Mature, sieved (<10 mm) home compost feedstock, characterized for pH, moisture (50-60%), volatile solids, and C/N ratio (20-30:1).
- Environmental Chamber or Insulated Room: To simulate seasonal temperature profiles (e.g., 20-35°C cycling).
B. Test Material Preparation:
- Prepare test materials as final products (e.g., bags, utensils) or films.
- Use a negative control (LDPE) and a positive control (cellulose filter paper).
- Weigh and optionally place materials in mesh bags for easier retrieval.
C. Test Execution (Based on AS 5810):
- Vessel Setup: Mix test material evenly into compost matrix at a concentration of ~1% (dry mass basis). Use multiple vessels for replicate and sacrificial time-point sampling.
- Incubation Conditions: Maintain moisture at 50-60% water holding capacity. Manually turn/mix contents weekly to simulate turning. Temperature follows a defined mesophilic profile (e.g., 4 weeks at 28±2°C, then 8 weeks at ambient 20-25°C).
- Monitoring: Weekly measurement of temperature, moisture, and O₂ concentration (>6%).
- Sampling: Sacrifice replicate vessels at defined intervals (e.g., 12, 24, 48 weeks).
- Analysis:
- Disintegration: Retrieve material remnants, clean, dry, and weigh. Calculate % disintegration:
[(Initial dry mass - Final dry mass) / Initial dry mass] * 100. - Biodegradation: Via mass balance (loss of organic carbon) or via CO₂ evolution in a respirometer setup adapted to the compost matrix.
- Ecotoxicity: Germination and growth tests (e.g., with cress seeds) on final compost to ensure no adverse effects.
- Disintegration: Retrieve material remnants, clean, dry, and weigh. Calculate % disintegration:
D. Pass Criteria (Exemplar from OK compost HOME):
- Disintegration: >90% fragmentation after 12 months, with no visible residues >2mm after screening.
- Biodegradation: >90% of absolute or relative to cellulose within 12 months.
- Heavy Metals: Below regulated limits.
- Eco-toxicity: No negative effect on plant growth.
Key Data & Interpretation
Table 2: Comparative Performance in Simulated Home Composting (12-Month Test)
| Material Type | Form | % Disintegration (12 mo.) | Visual Residue >2mm | Max Biodegradation (% vs. Cellulose) | Pass/Fail (Typical Home Cert.) |
|---|---|---|---|---|---|
| Cellulose Paper | Sheet | 100% | None | 100% (Ref.) | Pass |
| PHA Film | 40 µm film | 99 ± 1% | None | 98 ± 2% | Pass |
| PLA Cup | 200 µm wall | 15 ± 10% | Large fragments | 5 ± 3% | Fail |
| PBAT/Starch Blend | Shopping bag | 95 ± 4% | Minimal | 90 ± 5% | Pass* |
| Paper with Bio-coating | Cup lining | 100% | None | 95 ± 3% | Pass |
* May require specific thickness limits.
Visualizing the Validation Workflows
Diagram 1: ASTM D6691 Test Workflow (760px max)
Diagram 2: Home Compost Validation Parameters (760px max)
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 3: Key Reagent Solutions for Marine & Home Compost Testing
| Item Name / Kit | Primary Function | Application Context | Critical Notes |
|---|---|---|---|
| Natural Seawater Inoculum | Source of marine microbial consortium for biodegradation. | ASTM D6691 | Must be fresh (<36h), filtered; characterization of source advised. |
| Cellulose Microcrystalline (Positive Control) | Validates microbial activity in the test system. | ASTM D6691, Home Compost | Should show >70% mineralization in marine, >90% in compost. |
| Low-Density Polyethylene (Negative Control) | Confirms no abiotic CO₂ release is measured. | ASTM D6691, Home Compost | Should show <5% mineralization. |
| Artificial Seawater Salts (e.g., ASTM D1141) | Provides consistent baseline medium if natural seawater is compromised. | ASTM D6691 (if needed) | Must be supplemented with natural inoculum. |
| Mature Home Compost Inoculum | Source of mesophilic fungi & bacteria for home composting tests. | Home Compost Validation | Should be sieved, characterized for C/N, pH, and moisture. |
| CO₂ Absorption Solution (0.1-0.5 N NaOH) | Traps evolved CO₂ in respirometric systems for titration. | ASTM D6691, Respirometric Compost Tests | Must be used with CO₂-free air supply. |
| Barium Chloride Solution (0.5 M) | Precipitates carbonate before titration to determine trapped CO₂. | Titration Method for CO₂ | Ensures accuracy in two-stage titration. |
| Inorganic Nutrient Stock Solutions (N, P) | Prevents nutrient limitation from skewing biodegradation results. | ASTM D6691, Long-term Compost Tests | Ensure C:N:P > 40:10:1. |
| Seed Eco-toxicity Kit (e.g., Lepidium sativum) | Assesses phytotoxicity of final compost on plant growth. | Home Compost Validation (Pass/Fail) | Standardized by OECD 208. |
| Mesh Bags (Non-degradable, e.g., Nylon) | Holds test material for retrieval in disintegration tests. | Home Compost Validation | Mesh size must allow microbial access (e.g., 1mm). |
Correlating Standard Test Results with Advanced Characterization (DSC, GPC, SEM) for Mechanism Elucidation
Within the critical research domain of biopolymer biodegradability and compostability, a significant gap exists between the pass/fail outcomes of industry-standard tests and a fundamental understanding of the underlying degradation mechanisms. Standards such as ISO 14855 (aerobic biodegradation under controlled composting conditions) or ASTM D6400 provide essential compliance benchmarks but offer limited insight into chemical, morphological, and molecular-scale changes. This whitepaper posits that mechanistic elucidation—essential for material optimization and reliable prediction of environmental fate—requires the deliberate correlation of data from these standard tests with advanced analytical techniques, including Differential Scanning Calorimetry (DSC), Gel Permeation Chromatography (GPC), and Scanning Electron Microscopy (SEM).
The Analytical Triad: DSC, GPC, and SEM
Differential Scanning Calorimetry (DSC)
Function: Probes thermal transitions (glass transition Tg, melting Tm, crystallization Tc, and cold crystallization) and enthalpy changes. In biodegradation studies, changes in these parameters reflect alterations in polymer chain mobility, crystallinity, and thermal stability due to chain scission or cross-linking.
Protocol for Degradation Monitoring:
- Sample Preparation: Extract ~5-10 mg of material from a biodegrading sample at predefined time points (e.g., from an ISO 14855 test at 0, 30, 60, 90 days). Wash and dry to halt biological activity.
- Instrument Calibration: Calibrate the DSC for temperature and enthalpy using indium and zinc standards.
- Method: Perform a heat-cool-heat cycle under nitrogen purge (50 mL/min). Typical cycle: equilibrate at -50°C, heat to 250°C at 10°C/min (1st heating), cool to -50°C at 10°C/min, heat again to 250°C at 10°C/min (2nd heating).
- Data Analysis: Analyze the 2nd heating scan to determine Tg (midpoint), Tm (peak), and enthalpy of fusion (ΔHf). Percent crystallinity (Xc) is calculated via: Xc (%) = (ΔHf, sample / ΔHf, 100% crystalline) × 100.
Gel Permeation Chromatography (GPC)
Function: Measures the molecular weight distribution (MWD), number-average (Mn), and weight-average (Mw) molecular weights, and dispersity (Đ = Mw/Mn). It is the primary tool for quantifying chain scission, the dominant chemical mechanism in hydrolytic and enzymatic biodegradation.
Protocol for Degradation Monitoring:
- Sample Preparation: Dissolve precisely weighed (~5 mg) degraded polymer samples in the appropriate mobile phase (e.g., THF for PLA, DMAc with LiBr for polyesters) at a known concentration (~2 mg/mL). Filter through a 0.45 μm PTFE syringe filter.
- System Setup: Utilize a system with refractive index (RI) detection. Use a column set calibrated with narrow dispersity polystyrene or polymethyl methacrylate (PMMA) standards relevant to the polymer's hydrodynamic volume.
- Run Conditions: Isocratic elution at a flow rate of 1.0 mL/min at 30-40°C. Inject 100 μL of sample.
- Data Analysis: Process chromatograms to calculate Mn, Mw, and Đ. Monitor the shift of the MWD to lower molecular weights and the increase in Đ, which often indicates random chain scission before low-MW fragments are mineralized.
Scanning Electron Microscopy (SEM)
Function: Provides high-resolution, topographical images of the polymer surface. It visualizes physical degradation mechanisms such as biofilm formation, cracking, pitting, erosion, and the development of porosity.
Protocol for Degradation Monitoring:
- Sample Preparation: Sputter-coat the dried sample surfaces with a thin (~10 nm) layer of gold or gold/palladium using a sputter coater to ensure conductivity and prevent charging.
- Imaging: Mount samples on aluminum stubs using conductive carbon tape. Image at various magnifications (e.g., 500x to 20,000x) under high vacuum at an accelerating voltage appropriate for the material (typically 5-10 kV).
- Analysis: Qualitatively and quantitatively assess surface morphology changes. Use image analysis software to measure features like pore size distribution or surface roughness if an atomic force microscopy (AFM) attachment is available.
Correlation Framework and Data Interpretation
The core of mechanistic elucidation lies in temporally correlating data from standard biodegradation tests with the analytical triad's outputs. A typical workflow and logical relationship is shown below.
Multi-Technique Correlation Workflow for Biodegradation Analysis
Interpretative Guide:
- Lag Phase (Standards: Low CO₂ evolution; Advanced: SEM may show biofilm attachment. GPC shows minimal change. DSC may show slight Tg drop due to plasticization by water).
- Degradation Phase (Standards: Rapid CO₂ evolution; Advanced: GPD shows sharp decrease in Mn and broadening of Đ. SEM reveals surface pitting and erosion. DSC shows complex changes in crystallinity (Xc may increase then decrease).
- Plateau Phase (Standards: CO₂ evolution ceases; Advanced: GPC shows very low Mn or sample insoluble. SEM shows extensive fragmentation. DSC signals may be unmeasurable).
Data Synthesis and Tabulation
The table below synthesizes hypothetical but representative data from a polylactic acid (PLA) biodegradation study under composting conditions, illustrating the correlative approach.
Table 1: Correlated Data from a Simulated PLA Biodegradation Study (ISO 14855 Framework)
| Time Point (days) | Standard Test Result (Cumulative Biodegradation %) | GPC: Mn (kDa) | GPC: Dispersity (Đ) | DSC: Crystallinity (Xc, %) | SEM Morphological Description |
|---|---|---|---|---|---|
| 0 | 0 | 120 | 1.8 | 5 | Smooth, featureless surface |
| 15 | 5 | 95 | 2.1 | 18 | Initial biofilm coverage, minor pitting |
| 30 | 35 | 45 | 2.5 | 40 | Extensive pitting and cracking |
| 45 | 78 | 12 | 3.8 | 25 | Deep erosion, loss of structural integrity |
| 60 | >90 | <5 (soluble fraction) | N/A | N/A | Complete fragmentation, porous residue |
Data Interpretation: The correlation reveals a clear mechanism: An initial abiotic hydrolysis phase (days 0-15) causes slight chain scission (Mn↓), increasing chain mobility that allows chain reorganization and crystallinity increase (Xc↑). Accelerated enzymatic attack on amorphous regions follows (days 15-30), causing rapid Mn drop and visible pitting (SEM). The subsequent decrease in Xc (days 30-45) indicates the crystalline regions are now being attacked, leading to massive erosion and final mineralization.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 2: Key Reagents and Materials for Biodegradation Mechanism Studies
| Item | Function in Research | Example Application |
|---|---|---|
| Controlled Compost Inoculum | Provides the complex microbial consortium defined in ISO 14855. Essential for ecologically relevant biodegradation studies. | Inoculum preparation for aerobic biodegradation tests. |
| Enzyme Solutions (e.g., Proteinase K, Lipase) | Used in simplified, mechanistic in vitro studies to isolate and identify specific enzymatic degradation pathways. | Determining susceptibility to enzymatic hydrolysis. |
| Narrow Dispersity Polymer Standards | Critical for calibrating GPC systems to obtain accurate molecular weight data for the polymer of interest. | GPC calibration for PLA, PHA, PBS, etc. |
| High-Purity Solvents (HPLC/GPC Grade) | Ensure sample dissolution without introducing impurities that affect GPC columns or DSC thermal analysis. | Sample preparation for GPC (THF, DMAc, CHCl3). |
| Thermal Calibration Standards (Indium, Zinc) | Calibrate the temperature and enthalpy scales of the DSC, ensuring accuracy and inter-lab reproducibility. | DSC temperature/enthalpy calibration before sample runs. |
| Conductive Sputter Coating Material (Au/Pd) | Creates a thin, conductive layer on insulating polymer samples for SEM, preventing surface charging and enabling clear imaging. | Sample preparation for SEM imaging of biopolymers. |
| pH Buffers | Maintain specific pH conditions in in vitro degradation studies to isolate hydrolytic effects or optimize enzyme activity. | Studying pH-dependent hydrolysis (e.g., for polyester degradation). |
Moving beyond compliance to achieve true material innovation in biopolymers necessitates a deep mechanistic understanding. By systematically correlating the macroscopic, cumulative data from standard biodegradation tests with the molecular, thermal, and morphological fingerprints provided by DSC, GPC, and SEM, researchers can deconvolute complex degradation pathways. This correlative approach, framed within rigorous standards research, enables the rational design of polymers with predictable and tunable end-of-life behavior, accelerating the development of high-performance, truly sustainable materials.
Within the broader thesis on biopolymer biodegradability and compostability standards research, this guide elucidates the critical intersection of material science standards and regulatory submission strategies. For absorbable implants (e.g., sutures, stents, bone screws) and delivery vehicles (e.g., microparticles, hydrogels), compliance with established international standards provides a foundational framework for demonstrating safety and performance to the U.S. Food and Drug Administration (FDA) and under the European Union Medical Device Regulation (EU MDR 2017/745). This document serves as a technical guide for researchers navigating the complex evidentiary requirements for regulatory approval.
The Role of Key Standards in the Regulatory Framework
Regulatory bodies do not mandate specific standards, but conformity with harmonized standards provides a presumption of conformity to relevant General Safety and Performance Requirements (GSPRs under EU MDR) or safety and effectiveness principles (under FDA). Key standards address material characterization, degradation, and biological evaluation.
Material Characterization & Biodegradation Standards
Compliance provides quantitative data on the in vitro behavior of the material, which is essential for predicting in vivo performance.
Table 1: Core Material & Degradation Standards for Regulatory Submissions
| Standard Identifier | Title | Key Parameters Measured | Relevance to FDA/EU MDR |
|---|---|---|---|
| ISO 13781:2017 | Poly(L-lactide) resins and fabricated forms for surgical implants — In vitro degradation testing | Molecular weight loss, mass loss, thermal properties (DSC), mechanical property decay. | Demonstrates consistency of polymer resin and provides degradation kinetics for performance claims. |
| ASTM F1635 / ISO 13781 | Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants | Similar to ISO 13781. Provides detailed protocol for phosphate buffer solution (PBS) testing at 37°C. | Establishes a benchmark degradation profile; deviations in a novel polymer must be justified. |
| ISO 10993-13:2010 | Biological evaluation of medical devices — Part 13: Identification and quantification of degradation products from polymeric medical devices | Identification and quantification of leachables (monomers, oligomers, additives) in simulated body fluids. | Directly addresses safety (Cytotoxicity, Systemic Toxicity) by characterizing what is released into the body. |
| ISO 10993-14:2001 | Biological evaluation of medical devices — Part 14: Identification and quantification of degradation products from ceramics | Applies to ceramic or mineral-filled absorbable composites. | Supports evaluation of local tissue response to particulate debris. |
| ASTM F1980 | Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices | Applies to package integrity but methodology is adapted for accelerated in vitro degradation studies (e.g., elevated temperature). | Allows for real-time degradation predictions; crucial for establishing shelf-life and "use-by" dates. |
Biological Evaluation Standards (ISO 10993 Series)
A systematic biological evaluation plan, based on the device's nature and body contact, is mandatory.
Table 2: Essential ISO 10993 Parts for Absorbable Devices
| ISO 10993 Part | Focus Area | Critical Data for Submission |
|---|---|---|
| Part 1: Evaluation and testing | Risk management process | Justification for test selection based on device categorization. |
| Part 3: Tests for genotoxicity, carcinogenicity, and reproductive toxicity | Long-term toxicity potential | Required for permanent implantation or long-term absorbable devices (>30 days). |
| Part 4: Selection of tests for interactions with blood | Hemocompatibility | Critical for intravascular devices (e.g., absorbable stents). |
| Part 5: Tests for in vitro cytotoxicity | Cell death via extract or direct contact. | First-line screening test. Must be passed for all devices. |
| Part 6: Tests for local effects after implantation | In vivo tissue reaction (e.g., ISO 10993-6 implantation study). | Core study evaluating the local inflammatory response, fibrosis, and degradation profile in vivo. |
| Part 9: Framework for identification and quantification of potential degradation products | Links material degradation (Part 13) to biological risk. | Establishes the toxicological risk assessment (TRA) for identified leachables. |
| Part 11: Tests for systemic toxicity | Acute, subacute, subchronic, and chronic systemic effects. | Required for most implantable devices to assess effects on distant organs. |
Experimental Protocols: From Standard to Submission
Detailed Protocol:In VitroHydrolytic Degradation (Based on ASTM F1635/ISO 13781)
Objective: To characterize the mass loss, molecular weight loss, and mechanical property loss of a polylactide-based bone screw under simulated physiological conditions.
Materials & Reagents:
- Test specimens: Machined PLLA bone screws (n=60 per time point group).
- Control: Reference PLLA material of known in vivo performance.
- Degradation Medium: 0.1M Phosphate Buffered Saline (PBS), pH 7.4 ± 0.1, with 0.02% sodium azide (bacteriostatic agent).
- Equipment: Incubator/shaker at 37°C ± 1°C, vacuum desiccator, analytical balance (±0.01 mg), Gel Permeation Chromatography (GPC) system, Differential Scanning Calorimeter (DSC).
Procedure:
- Baseline Characterization: Weigh (M0), measure inherent viscosity/GPC for molecular weight (Mw0), and DSC for thermal properties (Tg0, Tm0, crystallinity) for a representative sample set (n=10).
- Immersion: Place individual specimens in labeled vials containing 10 mL of PBS per 100 mg of specimen. Ensure complete immersion.
- Incubation: Place vials in an incubator at 37°C ± 1°C with gentle agitation (e.g., 60 oscillations/min).
- Sampling & Medium Change: At predetermined time points (e.g., 1, 3, 6, 12, 18, 24 months), remove triplicate vials for each test and control group. Replace PBS weekly to maintain pH and ion concentration.
- Analysis: a. Mass Loss: Rinse specimens with deionized water, dry to constant mass in a vacuum desiccator (Mdry). Calculate percentage mass loss: [(M0 - Mdry)/M0] x 100. b. Molecular Weight: Analyze dried specimens via GPC to determine Mn and Mw relative to polystyrene standards. c. Thermal Properties: Analyze via DSC to track changes in glass transition (Tg), melting point (Tm), and crystallinity. d. Visual/Microscopic Inspection: Document surface erosion, cracking, or fragmentation.
Deliverable: A time-series dataset correlating property loss, crucial for predicting functional performance in vivo (e.g., screw holding power over 6 months).
Detailed Protocol:In VivoImplantation Study for Local Effects (Based on ISO 10993-6)
Objective: To evaluate the local tissue response to an absorbable hydrogel drug delivery vehicle in a subcutaneous rodent model.
Materials & Reagents:
- Test article: Sterile, finished hydrogel formulation (cylinders, 1mm diameter x 3mm length).
- Control articles: USP Negative Control Plastic (PE) and USP Positive Control Material (containing organotin).
- Animal Model: Female Sprague-Dawley rats (n=4 per article per time point, plus controls).
- Surgical supplies: Standard sterile surgical kit, isoflurane anesthesia, sutures.
- Histology: 10% Neutral Buffered Formalin, paraffin embedding microtome, H&E stain, specialized stains for macrophages (e.g., IBA1 immunohistochemistry).
Procedure:
- Implantation: Under aseptic technique and anesthesia, make a midline dorsal incision. Create subcutaneous pockets via blunt dissection. Randomly implant one specimen of each article (test, negative control, positive control) per animal, ensuring adequate separation. Suture the muscular layer and skin.
- Study Duration: Select time points relevant to degradation: e.g., 1, 4, 12, 26, and 52 weeks.
- Explant & Necropsy: At each time point, euthanize animals. Excise the implant site with a margin of surrounding tissue.
- Histopathological Processing: Fix tissue in formalin for 48h, process, and embed in paraffin. Section at 5µm thickness and stain with H&E.
- Evaluation: A board-certified pathologist, blinded to the groups, scores the tissue reaction per ISO 10993-6 criteria:
- Polymorphonuclear neutrophil count
- Lymphocyte count
- Plasma cell count
- Macrophage count
- Giant cell count
- Necrosis
- Fibrosis capsule thickness and quality
- Material degradation status (presence/amount of residual material)
Deliverable: A comparative histopathology report quantifying the inflammatory response over time, demonstrating the biocompatibility and absorption profile of the hydrogel.
Visualizing the Regulatory Strategy Workflow
Diagram 1 Title: Standard-Driven Path to Regulatory Submission
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Absorbable Implant R&D & Testing
| Item / Reagent Solution | Function / Application | Example/Supplier Note |
|---|---|---|
| Polymer Resin Standards | Reference materials for GPC calibration and comparative degradation studies. | Poly(L-lactide) of known Mw and D (e.g., from Polysciences, Corbion Purac). |
| Phosphate Buffered Saline (PBS), pH 7.4 | Standard in vitro degradation medium to simulate physiological ionic strength and pH. | Available sterile and in bulk from Thermo Fisher, Sigma-Aldrich. Must be azide-treated for long-term studies. |
| Simulated Body Fluids (SBF) | More advanced medium for bioresorbable ceramics (e.g., calcium phosphate) to assess apatite formation. | Prepared per Kokubo recipe or available as ready-to-use solutions (e.g., from Merck). |
| Mouse Fibroblast Cell Line (L929) | Standardized cell line for in vitro cytotoxicity testing (ISO 10993-5). | Available from ATCC; used in MTT, XTT, or direct contact assays. |
| USP Plastic Reference Standards | Mandatory controls for in vivo implantation studies (ISO 10993-6). | USP Class VI negative control polyethylene (RS) and positive control material. |
| Specific Histology Stains & Antibodies | Characterize the in vivo inflammatory and healing response. | H&E (general morphology), Masson's Trichrome (collagen/fibrosis), IBA1/CD68 antibodies (macrophages). |
| Sterile, Medical-Grade Solvents | For device fabrication (e.g., electrospinning, casting) and extraction studies. | High-purity, low-endotoxin dimethyl sulfoxide (DMSO), hexafluoroisopropanol (HFIP). |
| Gel Permeation Chromatography (GPC) System | Essential equipment for tracking hydrolytic chain scission and molecular weight loss over time. | Systems with RI and light scattering detectors (e.g., from Agilent, Waters) using appropriate columns (e.g., PLgel). |
Conclusion
Navigating the complex landscape of biopolymer biodegradability and compostability standards is crucial for advancing credible, sustainable biomedical innovations. A foundational understanding of degradation mechanisms informs the selection of appropriate ASTM, ISO, or EN methodologies. Rigorous application of these protocols, while mindful of common troubleshooting scenarios, generates robust data essential for material optimization. Ultimately, validation through comparative analysis and certification provides the critical link between laboratory research, regulatory approval, and trustworthy environmental or clinical claims. Future directions must focus on developing standards more predictive of specific in-vivo biological environments (e.g., tumor microenvironments) and harmonizing test protocols to accelerate the translation of next-generation, programmable biopolymers for drug delivery, tissue engineering, and implantable devices.